GMJ Medicine
eISSN : 2626-3041
Volume 2, Issue 2 (2023)
GMJM 2023, 2(2): 67-70 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2022/10/29 | Accepted: 2023/02/25 | Published: 2023/04/14
Received: 2022/10/29 | Accepted: 2023/02/25 | Published: 2023/04/14
How to cite this article
Nasseri A, Mahdavi F. Effect of Intravenous Infusion of Magnesium Sulfate on Opioid Use to Reduce Pain After Laparotomy in Patients with a History of Radiotherapy. GMJM 2023; 2 (2) :67-70
URL: http://gmedicine.de/article-2-188-en.html
URL: http://gmedicine.de/article-2-188-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
A. Nasseri *1, F. Mahdavi1
1- Tuberculosis and Lung Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Keywords:
| Abstract (HTML) (1563 Views)
Full-Text: (316 Views)
Introduction
Pain after major surgeries such as orthopedics, laparotomy, cardiovascular. As very severe pain has been reported by patients before surgery and most patients are stressed, afraid and afraid of how to control pain after major surgery. And if these pains are not well controlled after surgery, it will have a very negative effect on patient satisfaction [1]; Therefore, in relation to pain control and management after major surgery, physicians try different methods to find the best way to finally, in addition to reducing and managing postoperative pain, patient satisfaction, which is one of the principles of patient care [2, 3].
Many methods are used by surgeons before, during, and after surgery to control and manage pain, none of which has been able to replace opioid pain control; The use of opioids in addition to complications such as gastrointestinal complications, dependence, distress and respiratory apnea can have very adverse effects on laparotomy surgery due to nausea and vomiting. And the patient may need surgery again in the short or long term [4, 5].
Given that acute pain after laparotomy is considered as an unbearable pain for patients, management and control of postoperative pain with methods other than opioid use is very important due to their complications [6, 7]; The use of non-opioid oral and injectable analgesics before and during surgery is one of the methods that has been confirmed in most studies with positive effects, but there is still a method that can stop or minimize the amount of opioid use after surgery. Not expressed by studies; Therefore, finding such a method has been recommended by many researchers. One of the methods that researchers have recommended to conduct research on its effects on reducing opioid use after surgery is the use of intravenous infusion of magnesium sulfate around surgery [1].
Magnesium is a calcium antagonist and its effects on analgesia and anesthesia have recently been considered; The use of this drug in most studies has been suggested by researchers for reasons such as reduced pain intensity in the early hours after surgery and insignificant effects on hemodynamics of patients during surgery. However, there have been conflicting results in various studies on how to use this drug [8].
Due to the adverse effects of injectable opioids after surgery and the suggestions of many researchers to find a reliable way to replace opioid use and also the contradictory results of the most appropriate method of using magnesium sulfate to control and reduce postoperative pain, we decided to study The aim of the effect of intravenous infusion of magnesium sulfate on opioid use to reduce pain after laparotomy in patients with a history of radiotherapy.
Instrument and Methods
This study is a futuristic cross-sectional study that was conducted during 2019 (from the beginning to the end of 2019) in Imam Reza (AS) Hospital in Tabriz. 80 patients with inclusion criteria (age 20 to 60 years, SAS I-II class patients, candidate for thoracotomy, history of radiotherapy) and withdrawal (allergy to magnesium sulfate, liver and kidney failure, heart disease, duration of operation more than three hours). The four groups of 40 were divided into two groups of control and intervention by random and backward random block methods. After receiving the permits of the ethics committee number and referring to Imam Reza (AS) Hospital in Tabriz, in coordination with the hospital officials, sampling was done by available sampling method.
After explaining the objectives of the project to the patients, the informed consent form was completed by the samples and the intervention was performed. The intervention was that half an hour before anesthesia, 50mg/kg magnesium sulfate was injected into the patients as a bolus. After surgery and discharge from the recovery unit, the infusion of 500mg/hr magnesium sulfate was continued for 24 hours after surgery. It should be noted that for the control group, the amount of drug received in the intervention group was normal saline, similar to the group receiving magnesium sulfate. Induction of anesthesia was performed with fentanyl (1.5mg/kg), midazolam (0.01kg/kg), propofol (4mg/kg) and cis atracurium (0.1mg/kg). TIVA anesthesia was continued with propofol and remifentanil. At the end of the operation, muscle relaxation was restored with neostigmine (0.05mg/kg) and atropine (0.04mg/kg). Hemodynamic status (heart rate, blood pressure and arterial oxygen saturation) of all patients were measured and recorded before, during and after surgery, and during the operation, a change in hemodynamic status of more than 20% removed patients from the study. Opioid use in terms of mg of pethidine was also recorded in recovery and in the second, sixth, twelfth and twenty-fourth hours after surgery.
After collecting data and entering them in version 19 of SPSS 19 software, mean and standard deviation were used for descriptive statistics, Mann-Whitney u test and Fisher's exact test to compare independent groups. p<0.05 was considered significant.
Findings
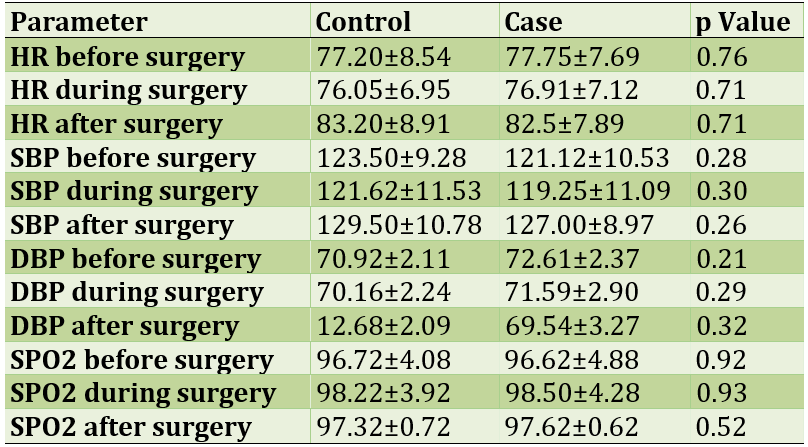
60% of patients were male and the rest were female. The mean age of patients in the intervention and control groups was 38.13±52.43 and 13.14±25.45 years, respectively. No statistically significant relationship was observed in the hemodynamic status of patients in both groups before, during and after surgery; The hemodynamic status of patients is given in Table 1.
Table 1) Hemodynamic status before, during and after surgery
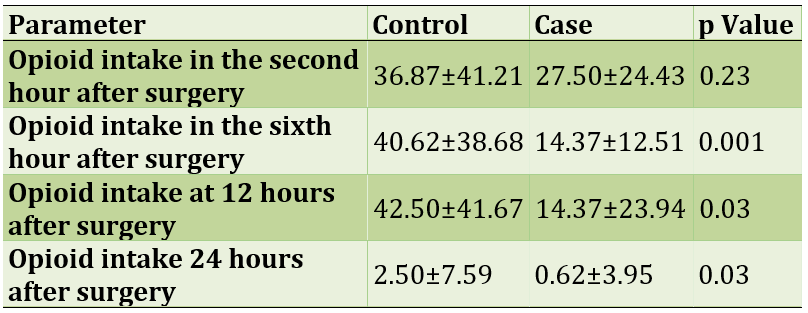
The rate of drug use in the intervention group was associated with a decrease over time, while no significant reduction was seen in the control group. Also, no statistically significant relationship was observed between the two groups only in the second hour after surgery and in the other hours. Opioid intake in mg of pethidine is shown in Table 2.
Table 2) Opioid intake in terms of mg of pethidine in the intervention and control groups
Discussion
The aim of this study was to evaluate the effect of intravenous infusion of magnesium sulfate on opioid use to reduce pain after laparotomy in patients with a history of radiotherapy. The results of a part of the study related to the hemodynamic status of patients showed no difference between the intervention and control groups before, during and after surgery; In other words, not all patients had a significant change in hemodynamic status. However, according to most studies, magnesium sulfate is the best choice in people who are not in a good condition because it has the least changes in hemodynamic status; However, in this study, not all patients underwent significant hemodynamic changes; The results of the present study are not consistent with similar studies in this field in terms of statistically significant differences between the two groups receiving magnesium sulfate and not receiving it [9, 10].
Opioid use in the intervention group has been significantly reduced over time, which indicates the positive effects of magnesium sulfate; The results of the present study are consistent with similar studies. The researchers say that in most studies, magnesium sulfate had a positive effect on reducing the need for opioids after surgery, but in no study did the need for opioids reach zero [11].
In the second hour after surgery, no difference was observed between the intervention and control groups in terms of opioid intake, which is not consistent with similar studies. The researchers say that in the first two hours after surgery, due to the lack of effects of anesthesia drugs and the effects of magnesium sulfate, the pain intensity and consequently the amount of opioid received is the lowest, and then the need for opioids is higher. The present study is inconsistent [12, 13].
On the other hand, in the sixth, twelfth and twenty-fourth hours after surgery, there was a statistically significant difference in opioid use between the control and intervention groups, which seems to be due to the analgesic effects of magnesium sulfate. The results of the present study are consistent with similar studies. One meta-analysis study found that magnesium sulfate could reduce the need for opioids in heart bypass surgery, but no study has reported zero [14].
Non-use of magnesium sulfate due to interference with neuromuscular relaxants during surgery is one of the weaknesses and limitations of the present study; Therefore, researchers recommend further studies to achieve the best way to use magnesium sulfate to achieve a suitable way to reduce the need for opioid after surgery to zero [15].
Conclusion
According to the results of the present study, it seems that this method of using magnesium sulfate, although it is effective in reducing the need for opioids in laparotomy patients with a history of radiotherapy, but can not reduce the need for opioids to zero.
Acknowledgements: None declared by the authors.
Ethical Permission: This study was confirmed by the ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1397.648).
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Pain after major surgeries such as orthopedics, laparotomy, cardiovascular. As very severe pain has been reported by patients before surgery and most patients are stressed, afraid and afraid of how to control pain after major surgery. And if these pains are not well controlled after surgery, it will have a very negative effect on patient satisfaction [1]; Therefore, in relation to pain control and management after major surgery, physicians try different methods to find the best way to finally, in addition to reducing and managing postoperative pain, patient satisfaction, which is one of the principles of patient care [2, 3].
Many methods are used by surgeons before, during, and after surgery to control and manage pain, none of which has been able to replace opioid pain control; The use of opioids in addition to complications such as gastrointestinal complications, dependence, distress and respiratory apnea can have very adverse effects on laparotomy surgery due to nausea and vomiting. And the patient may need surgery again in the short or long term [4, 5].
Given that acute pain after laparotomy is considered as an unbearable pain for patients, management and control of postoperative pain with methods other than opioid use is very important due to their complications [6, 7]; The use of non-opioid oral and injectable analgesics before and during surgery is one of the methods that has been confirmed in most studies with positive effects, but there is still a method that can stop or minimize the amount of opioid use after surgery. Not expressed by studies; Therefore, finding such a method has been recommended by many researchers. One of the methods that researchers have recommended to conduct research on its effects on reducing opioid use after surgery is the use of intravenous infusion of magnesium sulfate around surgery [1].
Magnesium is a calcium antagonist and its effects on analgesia and anesthesia have recently been considered; The use of this drug in most studies has been suggested by researchers for reasons such as reduced pain intensity in the early hours after surgery and insignificant effects on hemodynamics of patients during surgery. However, there have been conflicting results in various studies on how to use this drug [8].
Due to the adverse effects of injectable opioids after surgery and the suggestions of many researchers to find a reliable way to replace opioid use and also the contradictory results of the most appropriate method of using magnesium sulfate to control and reduce postoperative pain, we decided to study The aim of the effect of intravenous infusion of magnesium sulfate on opioid use to reduce pain after laparotomy in patients with a history of radiotherapy.
Instrument and Methods
This study is a futuristic cross-sectional study that was conducted during 2019 (from the beginning to the end of 2019) in Imam Reza (AS) Hospital in Tabriz. 80 patients with inclusion criteria (age 20 to 60 years, SAS I-II class patients, candidate for thoracotomy, history of radiotherapy) and withdrawal (allergy to magnesium sulfate, liver and kidney failure, heart disease, duration of operation more than three hours). The four groups of 40 were divided into two groups of control and intervention by random and backward random block methods. After receiving the permits of the ethics committee number and referring to Imam Reza (AS) Hospital in Tabriz, in coordination with the hospital officials, sampling was done by available sampling method.
After explaining the objectives of the project to the patients, the informed consent form was completed by the samples and the intervention was performed. The intervention was that half an hour before anesthesia, 50mg/kg magnesium sulfate was injected into the patients as a bolus. After surgery and discharge from the recovery unit, the infusion of 500mg/hr magnesium sulfate was continued for 24 hours after surgery. It should be noted that for the control group, the amount of drug received in the intervention group was normal saline, similar to the group receiving magnesium sulfate. Induction of anesthesia was performed with fentanyl (1.5mg/kg), midazolam (0.01kg/kg), propofol (4mg/kg) and cis atracurium (0.1mg/kg). TIVA anesthesia was continued with propofol and remifentanil. At the end of the operation, muscle relaxation was restored with neostigmine (0.05mg/kg) and atropine (0.04mg/kg). Hemodynamic status (heart rate, blood pressure and arterial oxygen saturation) of all patients were measured and recorded before, during and after surgery, and during the operation, a change in hemodynamic status of more than 20% removed patients from the study. Opioid use in terms of mg of pethidine was also recorded in recovery and in the second, sixth, twelfth and twenty-fourth hours after surgery.
After collecting data and entering them in version 19 of SPSS 19 software, mean and standard deviation were used for descriptive statistics, Mann-Whitney u test and Fisher's exact test to compare independent groups. p<0.05 was considered significant.
Findings
60% of patients were male and the rest were female. The mean age of patients in the intervention and control groups was 38.13±52.43 and 13.14±25.45 years, respectively. No statistically significant relationship was observed in the hemodynamic status of patients in both groups before, during and after surgery; The hemodynamic status of patients is given in Table 1.
Table 1) Hemodynamic status before, during and after surgery

The rate of drug use in the intervention group was associated with a decrease over time, while no significant reduction was seen in the control group. Also, no statistically significant relationship was observed between the two groups only in the second hour after surgery and in the other hours. Opioid intake in mg of pethidine is shown in Table 2.
Table 2) Opioid intake in terms of mg of pethidine in the intervention and control groups

Discussion
The aim of this study was to evaluate the effect of intravenous infusion of magnesium sulfate on opioid use to reduce pain after laparotomy in patients with a history of radiotherapy. The results of a part of the study related to the hemodynamic status of patients showed no difference between the intervention and control groups before, during and after surgery; In other words, not all patients had a significant change in hemodynamic status. However, according to most studies, magnesium sulfate is the best choice in people who are not in a good condition because it has the least changes in hemodynamic status; However, in this study, not all patients underwent significant hemodynamic changes; The results of the present study are not consistent with similar studies in this field in terms of statistically significant differences between the two groups receiving magnesium sulfate and not receiving it [9, 10].
Opioid use in the intervention group has been significantly reduced over time, which indicates the positive effects of magnesium sulfate; The results of the present study are consistent with similar studies. The researchers say that in most studies, magnesium sulfate had a positive effect on reducing the need for opioids after surgery, but in no study did the need for opioids reach zero [11].
In the second hour after surgery, no difference was observed between the intervention and control groups in terms of opioid intake, which is not consistent with similar studies. The researchers say that in the first two hours after surgery, due to the lack of effects of anesthesia drugs and the effects of magnesium sulfate, the pain intensity and consequently the amount of opioid received is the lowest, and then the need for opioids is higher. The present study is inconsistent [12, 13].
On the other hand, in the sixth, twelfth and twenty-fourth hours after surgery, there was a statistically significant difference in opioid use between the control and intervention groups, which seems to be due to the analgesic effects of magnesium sulfate. The results of the present study are consistent with similar studies. One meta-analysis study found that magnesium sulfate could reduce the need for opioids in heart bypass surgery, but no study has reported zero [14].
Non-use of magnesium sulfate due to interference with neuromuscular relaxants during surgery is one of the weaknesses and limitations of the present study; Therefore, researchers recommend further studies to achieve the best way to use magnesium sulfate to achieve a suitable way to reduce the need for opioid after surgery to zero [15].
Conclusion
According to the results of the present study, it seems that this method of using magnesium sulfate, although it is effective in reducing the need for opioids in laparotomy patients with a history of radiotherapy, but can not reduce the need for opioids to zero.
Acknowledgements: None declared by the authors.
Ethical Permission: This study was confirmed by the ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1397.648).
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Lirk P, Rathmell JR. Opioid-free anaesthesia: Con: it is too early to adopt opioid-free anaesthesia today. Eur J Anaesthesiol. 2019;36:250-4. [Link] [DOI:10.1097/EJA.0000000000000965]
2. Soghomonyan S, Stoicea N, Sandhu GS, Pasternak JJ, Bergese SD. The role of permissive and induced hypotension in current neuroanesthesia practice. Front Surg. 2017;4:1-10. [Link] [DOI:10.3389/fsurg.2017.00001]
3. Nielsen FH. Magnesium deficiency and increased inflammation: current perspectives. J Inflamm. Res. 2018;11:25-34. [Link] [DOI:10.2147/JIR.S136742]
4. Dunn LK, Durieux ME. Perioperative use of intravenous lidocaine. Anesthesiology. 2017;126:729-37. [Link] [DOI:10.1097/ALN.0000000000001527]
5. Chowdhury S, Chanda B. Sodium channels caught in the act. Science. 2019;363:1278-9. [Link] [DOI:10.1126/science.aaw8645]
6. Chang YC, Liu CL, Liu TP, Yang PS, Chen MJ, Cheng SP. Effect of perioperative intravenous lidocaine infusion on acute and chronic pain after breast surgery: A meta-analysis of randomized controlled trials. Pain Pract. 2017;17:336-43. [Link] [DOI:10.1111/papr.12442]
7. Kim MH, Lee KY, Park S, Kim SI, Park HS, Yoo YC. Effects of systemic lidocaine versus magnesium administration on postoperative functional recovery and chronic pain in patients undergoing breast cancer surgery: A prospective, randomized, double-blind, comparative clinical trial. PLoS One. 2017;12:e0173026. [Link] [DOI:10.1371/journal.pone.0173026]
8. Couceiro TCM, Lima LC, Burle LMC, Valença MM. Lidocaína intravenosa no tratamento da dor pós‐mastectomia: ensaio clínico aleatório encoberto placebo controladoIntravenous lidocaine for post‐mastectomy pain treatment: randomized, blind, placebo controlled clinical trial. Rev Bras Anestesiol. 2015;65:207-12. [Link] [DOI:10.1016/j.bjan.2014.05.016]
9. Weibel S, Jelting Y, Pace NL, Helf A, Eberhart LH, Hahnenkamp K, et al. Continuous intravenous perioperative lidocaine infusion for postoperative pain and recovery in adults. Cochrane Database Syst Rev. 2018;6:CD009642. [Link] [DOI:10.1002/14651858.CD009642.pub3]
10. Ho MLJ, Kerr SJ, Stevens J. Intravenous lidocaine infusions for 48 hours in open colorectal surgery: a prospective, randomized, double-blinded, placebo-controlled trial. Korean J Anesthesiol. 2018;71:57-65. [Link] [DOI:10.4097/kjae.2018.71.1.57]
11. Dewinter G, Moens P, Fieuws S, Vanaudenaerde B, de Velde MV, Rex S. Systemic lidocaine fails to improve postoperative morphine consumption, postoperative recovery and quality of life in patients undergoing posterior spinal arthrodesis. A double-blind, randomized, placebo-controlled trial. Br J Anaesth. 2017;118:576-85. [Link] [DOI:10.1093/bja/aex038]
12. Miró J, Castarlenas E, de la Vega R, Solé E, Tomé‐Pires C, Jensen MP, et al. Validity of three rating scales for measuring pain intensity in youths with physical disabilities. Eur J Pain. 2016;20:130-7. [Link] [DOI:10.1002/ejp.704]
13. Mendonca FT, Reis MC, Aguiar JA, Calvano LA. Systemic lidocaine for perioperative analgesia: A literature review. J Anesth Intensive Care Med. 2015;1:1-8. [Link] [DOI:10.19080/JAICM.2015.01.555551]
14. Rawal N. Current issues in postoperative pain management. Eur J Anaesthesiol. 2016;33:160-71. [Link] [DOI:10.1097/EJA.0000000000000366]
15. Ashmawi HA, Freire GMG. Sensibilização periférica e central. Rev Dor. 2016;17:31-4. [Portuguese [Link] [DOI:10.5935/1806-0013.20160044]









