GMJ Medicine
eISSN : 2626-3041
Volume 2, Issue 3 (2023)
GMJM 2023, 2(3): 101-107 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/01/6 | Accepted: 2023/05/28 | Published: 2023/07/20
Received: 2023/01/6 | Accepted: 2023/05/28 | Published: 2023/07/20
How to cite this article
Mohammadkhani Orouji F, Saeid Z. Medical Stability of Color Effects in Eyes. GMJM 2023; 2 (3) :101-107
URL: http://gmedicine.de/article-2-206-en.html
URL: http://gmedicine.de/article-2-206-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
F. Mohammadkhani Orouji *1, Z. Saeid2
1- Department of Psychology, Abarkouh Branch, Islamic Azad University, Abarkouh, Iran
2- Department of Psychology, University of Bangladesh University (BU), Bangladesh
2- Department of Psychology, University of Bangladesh University (BU), Bangladesh
| Abstract (HTML) (2638 Views)
Color temperature and color reproduction
The amount of color emitted by a hard object source is highly dependent on temperature. In fact, light emitted from such a source can be fully explained by the temperature (in Kelvin) at which the source operates. Such a criterion is called "color temperature". That is, in this measure we have a radiant black body [4, 18].
(Black Body Radiator) is gradually heated and step by step at different temperatures of radiation is analyzed by a spectrophotometer and determine the spectral and color properties of the emitted light (determine what wavelengths and in the emitted light Thus, at the end of our work, we have a list of different temperatures in Kelvin, each of which represents a type of light with a specific spectral characteristic. Below the criterion of "color temperature" is described in more detail and is discussed We start with the introduction of the radiant black body [2].
The test that determines the "color temperature" criterion has a simple method, as mentioned in the previous paragraph. We heat a hard object. The hard object heats up and emits light at any temperature with a specific color and spectral quality, so the temperature of the hard body per Kelvin unit can indicate the spectral properties and color quality of the emitted light.
In this experiment we need a kind of hard object that absorbs all the energy that is induced in it to eliminate any errors and inaccuracies in this experiment. The best and most suitable hard object for this purpose is a radiant black body [6].
The "black body" is a hollow black sphere made of steel with a small hole on it. The sphere is enclosed in a chamber of molten and boiling material. In such a way that the molten material enters from one side covers the entire surface of the ball and exits from the other side and heats the ball in a completely uniform manner. As the sphere heats up, little by little radiation, either invisible or visible, comes out of the small hole on the sphere. Regarding the structure and type of radiation of a black body, it is necessary to mention a few points [3]:
A. The black body is completely black. This is because if light shines on its outer or inner surface, it will be completely absorbed and will not be mistaken for light emitted by the black body itself during the experiment. In fact, since the inner surface of the sphere (black body) is completely concave and black, any light beam that enters the sphere through the hole will be absorbed immediately or after one or more reflections. As a result, the hole will be completely black, so when the walls of the sphere become hot, they emit radiant energy, which radiates out of the orbital hole. This output radiation, called blackbody radiation, is undoubtedly self-emitting and is not accompanied by any other unwanted radiation.
B. The rays emitted by an object (hard object) depend exactly on the "absolute temperature of the object" and the "radiative power of the object". Emissivity is the amount of radiation of an object per unit area. The reason that the black body is used to determine the Kelvin criterion is that the black body has full radiance and therefore the type of radiation emitted from this object depends solely on the "absolute temperature of the black body":
C. As the temperature of the black body increases, the properties of the radiant energy that are produced change systematically. This concept can be seen in the image below that as the temperature increases, the curves go higher, which indicates that the amount of energy emitted at each wavelength has increased. It is also evident that as the temperature increases, it forms the energy that emits the most. The shorter the wavelength (the peak of the curves is directed towards the shorter wavelengths). As the temperature increases, the position of the curved peak changes from the end of the long infrared wavelength to the end of the short ultraviolet wavelength. That part of the emitted energy that is perceived as light is a small part of the curve.
D. The black body produces continuous visible spectrum due to heating. In fact, there is a perfect relationship in this body, which is called the relationship between heat and light. According to this relation, the more the black body heats up, the lighter it produces, and the resulting visible spectrum, although it tends to radiate shorter wavelengths, is still continuous. Therefore, temperature numbers related to the color temperature criterion can be used and accurately used for those light sources in such a way that the sources in question are similar to a black body in terms of heat to light, they emit a continuous visible spectrum (such as tungsten lamps, solar lamps).
E. But there are some light sources that create a discontinuous visible spectrum and the color of the light emitted by them has nothing to do with their operating temperature (such as fluorescent lamps). In these cases, the concept of "correlated color temperature" is used, to determine the color temperature of one of these sources, we look at the temperature at which the black body produces visible light almost similar to the light from these sources, and as a result We consider that temperature as the "approximate color temperature" of the source. This concept also applies to monochrome light sources.
So, the concept of "color temperature" can be summarized as follows: When we say, for example, that the color temperature of this tungsten lamp is 3200 degrees Kelvin, it means that it emits a light whose spectral structure and color quality are similar to light. It is emitted by a black body at a temperature of 3200 degrees Kelvin [15].
That is, with a full understanding of "color temperature", numbers such as 3200 degrees Kelvin, when applied to a light, will be expressive symbols for the photographer, indicating what kind of light he is facing, what color quality and spectral structure, and to produce a color photograph with accurate translation. What kind of emulsion should the paints use? When the "color temperature" of a light source increases, the color of the light emitted from the source changes from red to white and then to blue. In fact, the color temperature of a light indicates the degree to which it tends to light - red or light - blue. The higher the tendency towards light-red color, the lower the color temperature will be, and the higher the tendency towards light-blue color, the higher the color temperature of light will be [6].
Often the actual color temperature of sources is different from their nominal temperature due to the influencers, so it is often necessary to use the actual color temperature of these sources using a color temperature sensor known as Kelvin Manter. (Color Temperature Meter=Kelvin Meter) measured.
The most common Kelvin meters are known as two-color Kelvin meters, which when measuring the color temperature of light, compare the water energy in the light with the red energy in it, and measure the result either directly as color temperature numbers (Kelvin numbers) or They present indirectly through charts. These types of Kelvin meters work well in daylight and tungsten (i.e., sources that produce a continuous spectrum are used), but to measure the approximate color temperature of sources with a discontinuous spectrum of three-color Kelvin meters that determine the temperature. Is used by comparing all three lights - red, green and blue in the light [1].
To create any color image in which the colors are translated correctly, the light-sensitive surface must match the light used in terms of color temperature. Otherwise the colors are translated incorrectly. That is, if the light used has a color temperature of 3200 degrees Kelvin, a light-sensitive surface should be used that is set to record such light. Only the human eye is an exception to this rule and can adapt to the temperature of different colors at any time, and as a result, the human eye in different lights often recognizes colors correctly, and even a combination of several lights with different color temperatures. Does not cause eye problems [1].
In photography, emulsions that are proportionate to the light must be used. If we use daylight, we must use emulsions that are set to a color temperature of 5500 degrees Kelvin (which is the temperature of daylight). Otherwise, incorrect translation of colors will take place. For this reason, in color photography, different emulsions are made, each of which is set to capture an image with a specific light (light with a specific color temperature). These emulsions include [17]:
A) Emulsion type A (Emulsion type A)
This emulsion is formulated for use with light with a color temperature of 3400 degrees Kelvin. This type of color film, which is used only in photography, is mostly produced for working with photographic flaps (Flood Lamp).
B) Emulsion type B (Emulsion type B).
This emulsion is formulated for use with light whose color temperature is 3200 degrees Kelvin. This type of color film is produced with a slight difference for both photography and cinema.
C) Day light emulsion-D
This emulsion is formulated for use with light whose color temperature is 5500 degrees Kelvin. Since the most famous light that has such a color temperature is daylight, this type of emulsion is called daylight emulsion. This type of color emulsion is produced with little difference for both photography and cinema.
In color filmmaking, another non-professional scale emulsion was used in the past, known as the G-type emulsion, which could provide almost accurate color translation if used with light at temperatures between 3200 °K and 5500 °K. In fact, this type of emulsion was designed to cover a variety of "color temperatures" from 3200 to 5500 degrees Kelvin, but this type of coating and adaptation was not perfect, and the color translation seemed almost correct, and therefore there was no professional application of this type of emulsion. This type of emulsion is not very popular today.
It is tried that the lamps and projectors that are produced have a color temperature commensurate with one of these two bases and the light-sensitive surfaces that are made (color emulsions for photography and filming. They are adjusted from these two bases (in video and digital cameras it is possible to choose both bases) thus all the tools produced (lamps, cameras, emulsions, related devices such as color correction filters and color in terms of) in terms of There is a certain standard that makes them very easy to work with. If there is no color temperature match between the light used and the emulsion used (emulsion or any kind of light-sensitive surface), the resulting image with color distortion and incorrect translation of colors. Will be found. If the light we use has a color temperature higher than the emulsion color temperature, a layer of blue will be added to all the colors of the resulting image, and if the light used has a color temperature lower than the emulsion temperature, an orange layer will be added to all the colors of the resulting image [15-19].
So the first step in capturing a color image correctly is to choose an emulsion that matches the color temperature of the scene with the existing light, but sometimes this is not possible and we are forced to use an emulsion that is disproportionate to the existing light or a light that is disproportionate to the existing emulsion. In these cases, color temperature filters can be used to compensate for this inconsistency. These filters fall into two families [13]:
Blue family
These are blue filters that increase the color temperature of light. For example, if we use light with a temperature of 3200 degrees Kelvin and type D emulsion (5500 degrees Kelvin), use a blue filter on the light or on the lens. The camera can ensure the correct translation of colors.
Orange family
These are orange filters that reduce the color temperature of light. For example, if we use light with a color temperature of 5500 degrees Kelvin and a type B emulsion (3200 degrees Kelvin), using an orange filter on the light or on the camera lens can ensure the correct translation of colors.
Of course, each of these two families includes different types of filters with different concentrations, some of which cause major changes and some minor changes in the color temperature of light sources.
Conclusion
In various imaging systems (photography, filming, video shooting, digital photography), among the possible color temperature sets, two color temperatures are accepted as two standard color temperature bases, which are: 3200 degrees Kelvin basis and 5500-degree basis.
Acknowledgements: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Full-Text: (1442 Views)
Introduction
When we enter a dark place from a very bright place, the perception of colors gradually decreases to the point that in very low light, the eye can not recognize other colors.
The maximum sensitivity of the eye in the green light area is 5500 angstroms and in weaker light the sensitivity will be higher in the 5000-angstrom area. Experiencing this phenomenon is not very difficult because it is well understood that in relatively low light blue color can still be seen as blue while red color will be suffocated. This phenomenon is used in photography and cinema, and in scenes intended to visualize the night, a predominant blue color covers most colors. For this reason, judging a color photo should be done in sufficient and complete light [1-3].
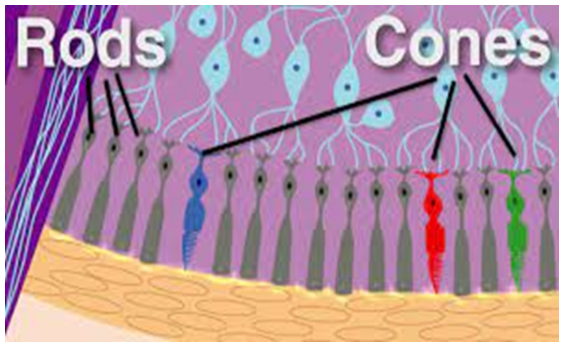
Figure 1) The Purkinje Effect: Why we can't see as many colors at night
Abnormal vision and congenital eye defects
A professional photographer, especially when working in color photography, must have excellent visual acuity and color recognition. In this regard, there are a number of congenital defects that need to be recognized [4-6]. People who are called "color blind" ACHROMATOPE do not recognize colors at all and see the world only in black and white, but fortunately this defect is extremely rare. In the eyes of people with DALTONISME, some colors are not visible or will be seen relatively weakly. It has different names depending on which of the main colors is difficult to distinguish. If it does not see red, it is "PROTANOPE", if it has a visual impairment in green, it is "DEUTERANOPE", and finally, if it is not sensitive to blue, it is "TRITANOPE".
Basically, a person with color blindness cannot be a good color photographer or color photographer. It is noteworthy that a large number of people are more or less suffering from this defect, they have some degree of color blindness without knowing it, and this complication is discovered during a general and systematic examination of the body. In terms of heredity, color blindness is also inherited and transmitted by the mother, and is usually more common in men [5-7].
Given this information, it is thought that those who want to pursue a career in photography and work in color and laboratory work need to have their eyes thoroughly examined by an ophthalmologist before making any decision. If they have this defect, they should refrain from choosing the photography profession. In the end, we conclude that everyone's vision is different from each other in seeing colors, and in addition, the way the colors are placed next to each other and the amount of brightness, etc., changes the color vision. Therefore, the judgment of different people, who firstly have different tastes and secondly may have some degree of color blindness, is different [9-11].
A- Spectrum of visible light and wavelengths
According to international standards, light is defined as: Light is the radiant energy that a human observer perceives through the sense of sight, which is produced by the stimulation of the retina.
Therefore, if we use this definition as the basis of our work, since from the set of electromagnetic spectra our eye can only see wavelengths of 720-380 nm, only this part of the radiant energy is called "light" and it is the only part that gives the visible attribute to It takes itself. As a result, the use of the two words "light and visible" for other parts of the electromagnetic spectrum should be avoided. For example, instead of ultraviolet light and infrared light, it is better to use the terms ultraviolet and infrared. For some rays outside the visible spectrum, terms such as black light or invisible light are sometimes used, which should be avoided because they cannot describe what kind of energy is intended and are confusing. And used the term "light" with any reasonable adjective only for the visible spectrum. Now, with this precondition, we examine the "nature of light".
Despite all the advances in science, scientists have not yet been able to come up with a comprehensive theory that defines the nature of light and justifies all the behaviors of light [12-15].
What is certain is that light moves, but the fundamental question remains, how does it move? And what does it take on when it moves? Over the past few centuries, attempts to answer these and similar questions have led to two well-known theories about light.
Particle Theory and Wave Theory
The truth is that neither of these two theories alone explains all of light behaviors and only explains some of them, and to escape this dilemma, scientists use a combination of both theories to justify light behaviors.
In order to take a picture of the objects and landscapes of this world, in addition to various possibilities, we need two basic tools: emulsion and radiant energy. All parts of the electromagnetic spectrum can be used as radiant energy to produce images, provided that appropriate emulsions are produced and used. For example, X-rays can record images of objects and subjects, provided that an emulsion that is sensitive to X-rays is prepared and used, and other steps are followed. This example also generalizes to infrared radiation and special infrared-sensitive emulsions, and images can be obtained with this tool [16].
Figure 2) The physics of color and light
But the most important thing about a typical photograph is that the image it presents of the world around it corresponds to our visual experience of seeing the world and its phenomena. For example, if we see the sky blue, we must see it in the photograph, and for that reason. Visible light and emulsion that is only sensitive to visible light are used to produce common photographs. The human retina responds only to visible light (780-380 nm) from different rays of the electromagnetic spectrum. That is why our visual experience of seeing world phenomena is formed by the presence of visible light. Conventional photography uses visible light and emulsion to which it is sensitive. Therefore, in order for the images obtained in this way to be in exact accordance with our visual experience, not only is it necessary to make an emulsion that is sensitive only to visible light, but it must also react to different parts of the visible light spectrum with the reaction of the human eye. Fully conform to these parts of the visible light spectrum [17-19].
The visible light spectrum ranges from about 400 to 700 nanometers. This means that between these two extremes there are infinite waves with different wavelengths greater than 400 nm and smaller than 700 nm. If we divide this spectrum into seven main parts and determine the exact amount of their wavelength with a spectrophotometer and then shine the relevant part on the human retina alone, one of the seven famous colors in the visible spectrum will be perceived. These seven colors from the short wavelength side (from the side of 400 nm) are: purple, blue, blue-green. Green. Yellow, orange, red.
White light will be perceived when all wavelengths between 400 and 700 nanometers are approximately equal to each other. In order to better understand the spectrum of visible light and its different parts, we can do the process mentioned in the previous paragraph in another way.
As we can see in the picture, if we pass a beam of white light through a glass prism, the light is decomposed into a set of colors that together comprise the visible spectrum. This decomposition of colors occurs because the different wavelengths of light each refract to different degrees they find [20].
Short-wavelength blue light fails more than long-wavelength green and red light. The result is a rainbow of colors ranging from dark purple to dark red. Experiments have shown that human observers can detect nearly 100 different colors within this range.
To know the reaction of the eye to different wavelengths of the visible spectrum, it is enough to look at the following curve. This curve shows the results of an experiment on 100 observers with natural color vision of light perception at different wavelengths of the visible spectrum. This curve is the average of all responses obtained from observers and shows the sensitivity of the eye to different wavelengths of light, and from this curve the following results can be derived [7]:
A. According to the curve, the sensitivity of the human eye to wavelengths shorter than 400 and longer than 700 nm is almost zero, and therefore these parts are invisible to the human eye.
B. The response of the eye to all wavelengths of the visible spectrum is not the same. This sensitivity has an upward trend from purple to green and a downward trend from green to red.
C. The reaction of the eye to light - green is more than all other parts of the spectrum, so if an equal physical number of different colors of light is presented to an observer, the middle part of the spectrum (green) is perceived most clearly. And at the end of the spectrum (blue and red) is much darker.
What is presented in this curve as a type of eye reaction to the visible spectrum. As an international standard reaction in light measurement.
(Standard Response Function) is accepted. Therefore, any measuring device used to measure light must have a Response Function or in other words a Sensitivity Function similar to this International Standard, and emulsions have a similar sensitivity to What is said in this curve about eye sensitivity should be relative to the wavelengths of the visible spectrum so that the resulting image is consistent with our visual experience of world phenomena and landscapes [19].
The main color indicators
Color has three distinct properties: Hue, Value, and Chroma. The "hue" of a color is its name, such as green or purple. We can also define Hugh by the location of the color in the visible spectrum [5].
"Value" refers to Hugh's relative intensity, such as light blue compared to dark blue or the relationship between light and darkness of a combined color. Brighter values are often called Tines, and darker values are called Shades.
"Pham" refers to the degree of saturation of the hue. We use these qualitative features to compare and select colors.
Because the color-receiving cone cells in the human retina are only sensitive to light-primary colors, the eye and brain only perceive colors by their dynamic comparison and contrast. We experience color because of its relationship to other colors [16].
Each of the wavelengths of the visible spectrum is seen in one of the colors purples, blue, blue-green, green, yellow, orange, red, and but this wide range can be summarized in three light-main colors.
These three light-colors, called "light-primary or primary colors," are [8]:
When we enter a dark place from a very bright place, the perception of colors gradually decreases to the point that in very low light, the eye can not recognize other colors.
The maximum sensitivity of the eye in the green light area is 5500 angstroms and in weaker light the sensitivity will be higher in the 5000-angstrom area. Experiencing this phenomenon is not very difficult because it is well understood that in relatively low light blue color can still be seen as blue while red color will be suffocated. This phenomenon is used in photography and cinema, and in scenes intended to visualize the night, a predominant blue color covers most colors. For this reason, judging a color photo should be done in sufficient and complete light [1-3].

Figure 1) The Purkinje Effect: Why we can't see as many colors at night
Abnormal vision and congenital eye defects
A professional photographer, especially when working in color photography, must have excellent visual acuity and color recognition. In this regard, there are a number of congenital defects that need to be recognized [4-6]. People who are called "color blind" ACHROMATOPE do not recognize colors at all and see the world only in black and white, but fortunately this defect is extremely rare. In the eyes of people with DALTONISME, some colors are not visible or will be seen relatively weakly. It has different names depending on which of the main colors is difficult to distinguish. If it does not see red, it is "PROTANOPE", if it has a visual impairment in green, it is "DEUTERANOPE", and finally, if it is not sensitive to blue, it is "TRITANOPE".
Basically, a person with color blindness cannot be a good color photographer or color photographer. It is noteworthy that a large number of people are more or less suffering from this defect, they have some degree of color blindness without knowing it, and this complication is discovered during a general and systematic examination of the body. In terms of heredity, color blindness is also inherited and transmitted by the mother, and is usually more common in men [5-7].
Given this information, it is thought that those who want to pursue a career in photography and work in color and laboratory work need to have their eyes thoroughly examined by an ophthalmologist before making any decision. If they have this defect, they should refrain from choosing the photography profession. In the end, we conclude that everyone's vision is different from each other in seeing colors, and in addition, the way the colors are placed next to each other and the amount of brightness, etc., changes the color vision. Therefore, the judgment of different people, who firstly have different tastes and secondly may have some degree of color blindness, is different [9-11].
A- Spectrum of visible light and wavelengths
According to international standards, light is defined as: Light is the radiant energy that a human observer perceives through the sense of sight, which is produced by the stimulation of the retina.
Therefore, if we use this definition as the basis of our work, since from the set of electromagnetic spectra our eye can only see wavelengths of 720-380 nm, only this part of the radiant energy is called "light" and it is the only part that gives the visible attribute to It takes itself. As a result, the use of the two words "light and visible" for other parts of the electromagnetic spectrum should be avoided. For example, instead of ultraviolet light and infrared light, it is better to use the terms ultraviolet and infrared. For some rays outside the visible spectrum, terms such as black light or invisible light are sometimes used, which should be avoided because they cannot describe what kind of energy is intended and are confusing. And used the term "light" with any reasonable adjective only for the visible spectrum. Now, with this precondition, we examine the "nature of light".
Despite all the advances in science, scientists have not yet been able to come up with a comprehensive theory that defines the nature of light and justifies all the behaviors of light [12-15].
What is certain is that light moves, but the fundamental question remains, how does it move? And what does it take on when it moves? Over the past few centuries, attempts to answer these and similar questions have led to two well-known theories about light.
Particle Theory and Wave Theory
The truth is that neither of these two theories alone explains all of light behaviors and only explains some of them, and to escape this dilemma, scientists use a combination of both theories to justify light behaviors.
In order to take a picture of the objects and landscapes of this world, in addition to various possibilities, we need two basic tools: emulsion and radiant energy. All parts of the electromagnetic spectrum can be used as radiant energy to produce images, provided that appropriate emulsions are produced and used. For example, X-rays can record images of objects and subjects, provided that an emulsion that is sensitive to X-rays is prepared and used, and other steps are followed. This example also generalizes to infrared radiation and special infrared-sensitive emulsions, and images can be obtained with this tool [16].

Figure 2) The physics of color and light
But the most important thing about a typical photograph is that the image it presents of the world around it corresponds to our visual experience of seeing the world and its phenomena. For example, if we see the sky blue, we must see it in the photograph, and for that reason. Visible light and emulsion that is only sensitive to visible light are used to produce common photographs. The human retina responds only to visible light (780-380 nm) from different rays of the electromagnetic spectrum. That is why our visual experience of seeing world phenomena is formed by the presence of visible light. Conventional photography uses visible light and emulsion to which it is sensitive. Therefore, in order for the images obtained in this way to be in exact accordance with our visual experience, not only is it necessary to make an emulsion that is sensitive only to visible light, but it must also react to different parts of the visible light spectrum with the reaction of the human eye. Fully conform to these parts of the visible light spectrum [17-19].
The visible light spectrum ranges from about 400 to 700 nanometers. This means that between these two extremes there are infinite waves with different wavelengths greater than 400 nm and smaller than 700 nm. If we divide this spectrum into seven main parts and determine the exact amount of their wavelength with a spectrophotometer and then shine the relevant part on the human retina alone, one of the seven famous colors in the visible spectrum will be perceived. These seven colors from the short wavelength side (from the side of 400 nm) are: purple, blue, blue-green. Green. Yellow, orange, red.
White light will be perceived when all wavelengths between 400 and 700 nanometers are approximately equal to each other. In order to better understand the spectrum of visible light and its different parts, we can do the process mentioned in the previous paragraph in another way.
As we can see in the picture, if we pass a beam of white light through a glass prism, the light is decomposed into a set of colors that together comprise the visible spectrum. This decomposition of colors occurs because the different wavelengths of light each refract to different degrees they find [20].
Short-wavelength blue light fails more than long-wavelength green and red light. The result is a rainbow of colors ranging from dark purple to dark red. Experiments have shown that human observers can detect nearly 100 different colors within this range.
To know the reaction of the eye to different wavelengths of the visible spectrum, it is enough to look at the following curve. This curve shows the results of an experiment on 100 observers with natural color vision of light perception at different wavelengths of the visible spectrum. This curve is the average of all responses obtained from observers and shows the sensitivity of the eye to different wavelengths of light, and from this curve the following results can be derived [7]:
A. According to the curve, the sensitivity of the human eye to wavelengths shorter than 400 and longer than 700 nm is almost zero, and therefore these parts are invisible to the human eye.
B. The response of the eye to all wavelengths of the visible spectrum is not the same. This sensitivity has an upward trend from purple to green and a downward trend from green to red.
C. The reaction of the eye to light - green is more than all other parts of the spectrum, so if an equal physical number of different colors of light is presented to an observer, the middle part of the spectrum (green) is perceived most clearly. And at the end of the spectrum (blue and red) is much darker.
What is presented in this curve as a type of eye reaction to the visible spectrum. As an international standard reaction in light measurement.
(Standard Response Function) is accepted. Therefore, any measuring device used to measure light must have a Response Function or in other words a Sensitivity Function similar to this International Standard, and emulsions have a similar sensitivity to What is said in this curve about eye sensitivity should be relative to the wavelengths of the visible spectrum so that the resulting image is consistent with our visual experience of world phenomena and landscapes [19].
The main color indicators
Color has three distinct properties: Hue, Value, and Chroma. The "hue" of a color is its name, such as green or purple. We can also define Hugh by the location of the color in the visible spectrum [5].
"Value" refers to Hugh's relative intensity, such as light blue compared to dark blue or the relationship between light and darkness of a combined color. Brighter values are often called Tines, and darker values are called Shades.
"Pham" refers to the degree of saturation of the hue. We use these qualitative features to compare and select colors.
Because the color-receiving cone cells in the human retina are only sensitive to light-primary colors, the eye and brain only perceive colors by their dynamic comparison and contrast. We experience color because of its relationship to other colors [16].
Each of the wavelengths of the visible spectrum is seen in one of the colors purples, blue, blue-green, green, yellow, orange, red, and but this wide range can be summarized in three light-main colors.
These three light-colors, called "light-primary or primary colors," are [8]:
- Red (Red)
- Green
- Blue
Which is called RGB for short.
These three light-colors can be combined in pairs or all three together, this mixture is called "mixed" or "additive". From the mixture of equal blue and red, light-color "Magenta" is produced. From the equal combination of blue and green, light-color "cyan" is produced and from the equal combination of green and red, light-color "yellow" is produced. These three lights - the new color - are called "light - secondary colors" [8]:
These three light-colors can be combined in pairs or all three together, this mixture is called "mixed" or "additive". From the mixture of equal blue and red, light-color "Magenta" is produced. From the equal combination of blue and green, light-color "cyan" is produced and from the equal combination of green and red, light-color "yellow" is produced. These three lights - the new color - are called "light - secondary colors" [8]:
- Yellow
- Magenta
- Syan (Cyan)
Which is called YMC for short.
These three light-colors can be combined in pairs or all three together, this mixture is called "subtractive" or subtractive.
If all three light-complementary colors are combined with equal intensity, black is produced. From the equal combination of cyan and magenta, light-blue color is obtained.
From the equal combination of cyan and yellow, light-green color is created, and from the equal combination of yellow and magenta, light-red color is created. Thus these 4 lights [11]:
These three light-colors can be combined in pairs or all three together, this mixture is called "subtractive" or subtractive.
If all three light-complementary colors are combined with equal intensity, black is produced. From the equal combination of cyan and magenta, light-blue color is obtained.
From the equal combination of cyan and yellow, light-green color is created, and from the equal combination of yellow and magenta, light-red color is created. Thus these 4 lights [11]:
- Primary and secondary colors - are complementary in pairs (complementary colors to both light - colors that produce light - white in combination with each other, is called white), "yellow and blue", "magnets and "Green" and "Red and Cyan" are complementary.
- To better understand these 6 light-colors, they can be designed on a triangle called the "Maxwell Triangle" so that the three lights - the primary color on the three vertices of the triangle and the three lights - the secondary color on the three sides of the triangle.
- The light-color on each side is complemented by the light-color on top of the opposite side. The figure below shows this triangle.
- The degree and purity of light - the resulting colors relative to the degree of purity of light - are dependent colors that combine. Red and blue will not produce magnets unless the ratio and purity of these are equal and constant. By slightly changing the volume of the primary colors, a range of light-secondary colors with different degrees of saturation is obtained.
Color temperature and color reproduction
The amount of color emitted by a hard object source is highly dependent on temperature. In fact, light emitted from such a source can be fully explained by the temperature (in Kelvin) at which the source operates. Such a criterion is called "color temperature". That is, in this measure we have a radiant black body [4, 18].
(Black Body Radiator) is gradually heated and step by step at different temperatures of radiation is analyzed by a spectrophotometer and determine the spectral and color properties of the emitted light (determine what wavelengths and in the emitted light Thus, at the end of our work, we have a list of different temperatures in Kelvin, each of which represents a type of light with a specific spectral characteristic. Below the criterion of "color temperature" is described in more detail and is discussed We start with the introduction of the radiant black body [2].
The test that determines the "color temperature" criterion has a simple method, as mentioned in the previous paragraph. We heat a hard object. The hard object heats up and emits light at any temperature with a specific color and spectral quality, so the temperature of the hard body per Kelvin unit can indicate the spectral properties and color quality of the emitted light.
In this experiment we need a kind of hard object that absorbs all the energy that is induced in it to eliminate any errors and inaccuracies in this experiment. The best and most suitable hard object for this purpose is a radiant black body [6].
The "black body" is a hollow black sphere made of steel with a small hole on it. The sphere is enclosed in a chamber of molten and boiling material. In such a way that the molten material enters from one side covers the entire surface of the ball and exits from the other side and heats the ball in a completely uniform manner. As the sphere heats up, little by little radiation, either invisible or visible, comes out of the small hole on the sphere. Regarding the structure and type of radiation of a black body, it is necessary to mention a few points [3]:
A. The black body is completely black. This is because if light shines on its outer or inner surface, it will be completely absorbed and will not be mistaken for light emitted by the black body itself during the experiment. In fact, since the inner surface of the sphere (black body) is completely concave and black, any light beam that enters the sphere through the hole will be absorbed immediately or after one or more reflections. As a result, the hole will be completely black, so when the walls of the sphere become hot, they emit radiant energy, which radiates out of the orbital hole. This output radiation, called blackbody radiation, is undoubtedly self-emitting and is not accompanied by any other unwanted radiation.
B. The rays emitted by an object (hard object) depend exactly on the "absolute temperature of the object" and the "radiative power of the object". Emissivity is the amount of radiation of an object per unit area. The reason that the black body is used to determine the Kelvin criterion is that the black body has full radiance and therefore the type of radiation emitted from this object depends solely on the "absolute temperature of the black body":
C. As the temperature of the black body increases, the properties of the radiant energy that are produced change systematically. This concept can be seen in the image below that as the temperature increases, the curves go higher, which indicates that the amount of energy emitted at each wavelength has increased. It is also evident that as the temperature increases, it forms the energy that emits the most. The shorter the wavelength (the peak of the curves is directed towards the shorter wavelengths). As the temperature increases, the position of the curved peak changes from the end of the long infrared wavelength to the end of the short ultraviolet wavelength. That part of the emitted energy that is perceived as light is a small part of the curve.
D. The black body produces continuous visible spectrum due to heating. In fact, there is a perfect relationship in this body, which is called the relationship between heat and light. According to this relation, the more the black body heats up, the lighter it produces, and the resulting visible spectrum, although it tends to radiate shorter wavelengths, is still continuous. Therefore, temperature numbers related to the color temperature criterion can be used and accurately used for those light sources in such a way that the sources in question are similar to a black body in terms of heat to light, they emit a continuous visible spectrum (such as tungsten lamps, solar lamps).
E. But there are some light sources that create a discontinuous visible spectrum and the color of the light emitted by them has nothing to do with their operating temperature (such as fluorescent lamps). In these cases, the concept of "correlated color temperature" is used, to determine the color temperature of one of these sources, we look at the temperature at which the black body produces visible light almost similar to the light from these sources, and as a result We consider that temperature as the "approximate color temperature" of the source. This concept also applies to monochrome light sources.
So, the concept of "color temperature" can be summarized as follows: When we say, for example, that the color temperature of this tungsten lamp is 3200 degrees Kelvin, it means that it emits a light whose spectral structure and color quality are similar to light. It is emitted by a black body at a temperature of 3200 degrees Kelvin [15].
That is, with a full understanding of "color temperature", numbers such as 3200 degrees Kelvin, when applied to a light, will be expressive symbols for the photographer, indicating what kind of light he is facing, what color quality and spectral structure, and to produce a color photograph with accurate translation. What kind of emulsion should the paints use? When the "color temperature" of a light source increases, the color of the light emitted from the source changes from red to white and then to blue. In fact, the color temperature of a light indicates the degree to which it tends to light - red or light - blue. The higher the tendency towards light-red color, the lower the color temperature will be, and the higher the tendency towards light-blue color, the higher the color temperature of light will be [6].
Often the actual color temperature of sources is different from their nominal temperature due to the influencers, so it is often necessary to use the actual color temperature of these sources using a color temperature sensor known as Kelvin Manter. (Color Temperature Meter=Kelvin Meter) measured.
The most common Kelvin meters are known as two-color Kelvin meters, which when measuring the color temperature of light, compare the water energy in the light with the red energy in it, and measure the result either directly as color temperature numbers (Kelvin numbers) or They present indirectly through charts. These types of Kelvin meters work well in daylight and tungsten (i.e., sources that produce a continuous spectrum are used), but to measure the approximate color temperature of sources with a discontinuous spectrum of three-color Kelvin meters that determine the temperature. Is used by comparing all three lights - red, green and blue in the light [1].
To create any color image in which the colors are translated correctly, the light-sensitive surface must match the light used in terms of color temperature. Otherwise the colors are translated incorrectly. That is, if the light used has a color temperature of 3200 degrees Kelvin, a light-sensitive surface should be used that is set to record such light. Only the human eye is an exception to this rule and can adapt to the temperature of different colors at any time, and as a result, the human eye in different lights often recognizes colors correctly, and even a combination of several lights with different color temperatures. Does not cause eye problems [1].
In photography, emulsions that are proportionate to the light must be used. If we use daylight, we must use emulsions that are set to a color temperature of 5500 degrees Kelvin (which is the temperature of daylight). Otherwise, incorrect translation of colors will take place. For this reason, in color photography, different emulsions are made, each of which is set to capture an image with a specific light (light with a specific color temperature). These emulsions include [17]:
A) Emulsion type A (Emulsion type A)
This emulsion is formulated for use with light with a color temperature of 3400 degrees Kelvin. This type of color film, which is used only in photography, is mostly produced for working with photographic flaps (Flood Lamp).
B) Emulsion type B (Emulsion type B).
This emulsion is formulated for use with light whose color temperature is 3200 degrees Kelvin. This type of color film is produced with a slight difference for both photography and cinema.
C) Day light emulsion-D
This emulsion is formulated for use with light whose color temperature is 5500 degrees Kelvin. Since the most famous light that has such a color temperature is daylight, this type of emulsion is called daylight emulsion. This type of color emulsion is produced with little difference for both photography and cinema.
In color filmmaking, another non-professional scale emulsion was used in the past, known as the G-type emulsion, which could provide almost accurate color translation if used with light at temperatures between 3200 °K and 5500 °K. In fact, this type of emulsion was designed to cover a variety of "color temperatures" from 3200 to 5500 degrees Kelvin, but this type of coating and adaptation was not perfect, and the color translation seemed almost correct, and therefore there was no professional application of this type of emulsion. This type of emulsion is not very popular today.
It is tried that the lamps and projectors that are produced have a color temperature commensurate with one of these two bases and the light-sensitive surfaces that are made (color emulsions for photography and filming. They are adjusted from these two bases (in video and digital cameras it is possible to choose both bases) thus all the tools produced (lamps, cameras, emulsions, related devices such as color correction filters and color in terms of) in terms of There is a certain standard that makes them very easy to work with. If there is no color temperature match between the light used and the emulsion used (emulsion or any kind of light-sensitive surface), the resulting image with color distortion and incorrect translation of colors. Will be found. If the light we use has a color temperature higher than the emulsion color temperature, a layer of blue will be added to all the colors of the resulting image, and if the light used has a color temperature lower than the emulsion temperature, an orange layer will be added to all the colors of the resulting image [15-19].
So the first step in capturing a color image correctly is to choose an emulsion that matches the color temperature of the scene with the existing light, but sometimes this is not possible and we are forced to use an emulsion that is disproportionate to the existing light or a light that is disproportionate to the existing emulsion. In these cases, color temperature filters can be used to compensate for this inconsistency. These filters fall into two families [13]:
Blue family
These are blue filters that increase the color temperature of light. For example, if we use light with a temperature of 3200 degrees Kelvin and type D emulsion (5500 degrees Kelvin), use a blue filter on the light or on the lens. The camera can ensure the correct translation of colors.
Orange family
These are orange filters that reduce the color temperature of light. For example, if we use light with a color temperature of 5500 degrees Kelvin and a type B emulsion (3200 degrees Kelvin), using an orange filter on the light or on the camera lens can ensure the correct translation of colors.
Of course, each of these two families includes different types of filters with different concentrations, some of which cause major changes and some minor changes in the color temperature of light sources.
Conclusion
In various imaging systems (photography, filming, video shooting, digital photography), among the possible color temperature sets, two color temperatures are accepted as two standard color temperature bases, which are: 3200 degrees Kelvin basis and 5500-degree basis.
Acknowledgements: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153-63. [Link] [DOI:10.1016/S0928-4680(00)00053-5]
2. Kapur I, Macdonaald RL. Rapid seizure-induced reduction of benzodiazepine and zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997:17(19);153-4. [Link] [DOI:10.1523/JNEUROSCI.17-19-07532.1997]
3. Kaputlu I, Uzbay T. l-Name inhibits pentylenetetrazole and strychnine-induced seizures in mice. Brain Res. 1997;753(1):98-101. [Link] [DOI:10.1016/S0006-8993(96)01496-5]
4. Ravi K, Rajasekaran S, Subramanian S. antihyperlipidemic effect of eugenia jambolana seed kernel on streptozotocin-induced diabetes in rats. FCT. 2005;43(9):1433-9. [Link] [DOI:10.1016/j.fct.2005.04.004]
5. Kasper K, Braunwald E, Fauci A, Houser S, Longo D, Jamson JL. Harisons Principale of Internal medicine. 16th ed. New York: MC Grow; 2002. [Link]
6. Kaviarasan K, Arjunan MM, Pugalendi KV. Lipid profile, oxidant-antioxidant status and glycoprotein components in hyperlipidemic patients with/without diabetes. Clin Chim Acta. 2005;362(1-);49-56. [Link] [DOI:10.1016/j.cccn.2005.05.010]
7. Khaksari M, Mahmoudi M, Ferdowsi F, Asadi Karam G, Shariati M. the effect of trifluoperazine on increased vascular permeability in an experimental model of chronic diabetic rat. J Physiol Pharmacol. 2005;9(1):47-55. [Persian] [Link]
8. Khine H, Weiss D, Graber N, Hoffman RS, Esteban-Cruciani N, Avner JR. A cluster of children with seizures caused by camphor poisoning. Pediatrics. 2009;123(5):1269-72. [Link] [DOI:10.1542/peds.2008-2097]
9. Kilian M, Freg HH. Central monoamines and convulsive thresholds in mice and rats. Neurpharmacology. 1973;12(7):681-92. [Link] [DOI:10.1016/0028-3908(73)90121-4]
10. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care. 1998;21(9):1414-31. [Link] [DOI:10.2337/diacare.21.9.1414]
11. Janusz W, Kleinork Z. The role of the central serotonergic system in pilocarpine-induced seizures: Receptor mechanisms. Neurosci Res. 1989;7(2):144-53. [Link] [DOI:10.1016/0168-0102(89)90054-0]
12. Knekt P, Beunanen A, Jarvinen R, Seppane R, Helio VM, Aroma A. antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139(12):1180-9. [Link] [DOI:10.1093/oxfordjournals.aje.a116964]
13. Kumarasamy YY, Nahar L, Byres M, Delazar A, Sarker SD. The assessment of biological activities associated with the major constituents of the methanol extract of 'wild carrot' (Daucus carotaL.) seeds. J Herb Pharmacother. 2005;5(1):61-72. [Link] [DOI:10.1300/J157v05n01_07]
14. Kuzuya T, Nakagawa S, Satoh J, Kanazawa S, Iwamota Y, Kobayashi M, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55(1):65-85. [Link] [DOI:10.1016/S0168-8227(01)00365-5]
15. Lee J, Sparrow D, Vokonas PS, Landsberg L, Weiss ST. Uric acid and coronary heart disease risk: evidence for a role of uric acid in the obesity-insulin resistance syndrome: The normative aging study. Am J Epidemiol. 1995;142(3):228-94. [Link] [DOI:10.1093/oxfordjournals.aje.a117634]
16. Lehto S, Niskanen L, Ronnemaa T, Laakso M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1998;29(3):635-9. [Link] [DOI:10.1161/01.STR.29.3.635]
17. Loh KC, Leow MK. Current therapeutic strategies for type 2 diabetes mellitus. Ann Acad Med Singap. 2002;31(6):722-9. [Link]
18. Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. 1997;46(2):538-42. [Link] [DOI:10.2337/diab.46.2.S38]
19. Macdonald PE, Wheeler MB. Voltage-dependent K+ channels in pancreatic beta cells: Role, regulation and potential as therapeutic targets. Diabetologia. 2005;46:1046-62. [Link] [DOI:10.1007/s00125-003-1159-8]
20. Majumdar UK, Grupta M,. Datro VJ. Studies on antifertility of methanolic extract of Daucus carota Linn. seeds. Seeds Indian J Nat Prod. 1998;14(22):33-7. [Link]









