GMJ Medicine
eISSN : 2626-3041
Volume 2, Issue 4 (2023)
GMJM 2023, 2(4): 127-131 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/05/1 | Accepted: 2023/11/5 | Published: 2023/12/14
Received: 2023/05/1 | Accepted: 2023/11/5 | Published: 2023/12/14
How to cite this article
Saboktakin L. Relationship Between Hypothyroidism and Body Mass Index in Children. GMJM 2023; 2 (4) :127-131
URL: http://gmedicine.de/article-2-208-en.html
URL: http://gmedicine.de/article-2-208-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
L. Saboktakin *
“Tuberculosis and Lung Disease Research Center” and “Department of Otorhinolaryngology”, Tabriz University of Medical Sciences, Tabriz, Iran
Keywords:
| Abstract (HTML) (1516 Views)
Full-Text: (661 Views)
Introduction
Most thyroid diseases seen in adults also occur in children. Although the treatment of these diseases in children is slightly different from adults, the principles in us are the same in both groups. Congenital hypothyroidism, congenital goiter, Hashimoto's thyroiditis and Graves' disease are the most important thyroid diseases in children [1, 2].
The normal growth and development of the fetal thyroid gland and the production of thyroid hormone are vital for the development of the brain during and after fetal life. The concentration of thyroid hormones in the fetal circulation is low in the first half of pregnancy. During this period, the fetus is completely dependent on maternal thyroid hormone, which passes through the placenta and reaches the fetus. The production of thyroid hormones (thyroxine and to a lesser extent triiodothyronine) in the fetal thyroid gland begins at 12 weeks of gestation [3, 4].
The prevalence of this disease varies in different countries and from one case in 3500 to 4000 live births have been reported. Studies in Iran have estimated this rate at one in 1000 live births. Thyroid hormone deficiency is associated with a wide range of neuropsychiatric disorders during the fetal life and the first year after birth. Congenital hypothyroidism can be primary (hypothyroidism), secondary (hypopharyngeal), and tertiary (hypothalamic) [5].
Congenital hypothyroidism can be transient or permanent. The primary and permanent type is the most common type of congenital hypothyroidism in infants. About 80 to 90 percent of the cause of permanent congenital hypothyroidism is monocytoma and is due to an evolutionary disorder of the thyroid gland, meaning that the thyroid gland may not be present at all or may be smaller than normal. Abnormal growth of the thyroid gland is also a type of developmental defect [6]. The ectopic thyroid gland may be located anywhere from behind the tongue to its normal location in the front of the neck. 10 to 20% of cases are due to problems in the production of thyroid hormones [7]. These disorders are inherited and are more common in consanguineous marriages. Permanent hypothyroidism of central origin (disorder of the hypothalamus or hypophysis) is very rare and usually affects one in 50,000 infants. This type of congenital hypothyroidism is always accompanied by a deficiency of other hormones in the pituitary gland or the hypo to the mouse. Transient types of congenital hypothyroidism are prevalent in areas with moderate to severe iodine deficiency [8].
Only a handful of infants with congenital hypothyroidism show clinical signs and symptoms of hypothyroidism at birth, including delayed birth, macular degeneration, jaundice, and delayed skeletal system development. A small percentage of infants have goiter. Other signs and symptoms include malnutrition, hypothermia, constipation, prolonged jaundice, abdominal distention, umbilical hernia, dry skin, large tongue, and heavy crying [6].
Because most babies with congenital hypothyroidism do not show signs and symptoms of hypothyroidism at birth, a screening program is recommended to diagnose the disease. Today, in most countries of the world, including Iran, a screening program is performed by taking a blood sample 2 to 5 days after birth from the heel. In this blood sample, the levels of TSH and T4 are measured. If they are abnormal, the baby will be treated with levothyroxine tablets. Early diagnosis and early treatment prevent the baby from developing mental retardation and promote normal growth [9].
In some studies, obesity has been linked to pediatric thyroid disorders; This relationship can lead to many risks in children; Because both of these diseases have side effects that can occur more severely together; High blood pressure, disorders of the immune system, as well as cardiovascular disease occur in children who are obese and have thyroid disorders 16 times more often than normal children; The aim of this study was the relationship between hypothyroidism and body mass index in children.
Instrument and Methods
Study design
This is a cross-sectional descriptive study that was conducted during 2019 in hospital centers affiliated to Tabriz University of Medical Sciences with the participation of 100 people under 12 years old. The sampling method was available and available among children referred to the clinics of hospitals in Tabriz. All participants were assessed based on inclusion and exclusion criteria.
Inclusion criteria included age under 12, consent to participate in the study, ability to participate in the study, and comfort in performing the relevant tests; Exclusion criteria included vitamin D intake, calcium intake, intake of any multivitamin, musculoskeletal disorders and cancers, and receiving chemotherapy drugs.
Procedure
All participants in the study were examined for demographic factors. These factors included age, gender, education, history of diabetes mellitus, and history of uncontrolled hypertension. Then, with the least amount of clothing, their weight was measured with the help of a scale (with an accuracy of half a kilogram) made in Germany (Seca). After that, their height was measured with the help of a meter with an accuracy of half a centimeter. All participants were leaning against the wall, standing upright on the floor, and their height was measured. Then their body mass index was measured. Thyroid tests were then taken from all participants on an empty stomach. The fasting thyroid test is done by taking a blood sample in a blood laboratory, and this test is done for the baby through the baby's heel. In a thyroid test, the amount of T4, T3, and TSH is checked to determine the type of thyroid of the affected person, whether the thyroid is overactive or underactive. In general, thyroid testing does not require special preparation and work, only fasting is important in thyroid testing. It should be noted that eating my job, peanuts, and cabbage, which cause iodine excretion, were banned for three days before the test. The children were then exposed to both normal and abnormal levels based on total thyroxine (T4), free thyroxine (TF4), complete triiodothyronine (T3) and free triiodothyronine. Children's body mass index was also categorized as: lean/abnormal weight loss, lean, normal weight, somewhat obese, obese, high-risk obesity, and very high-risk obesity/abnormal obesity.
Ethical considerations
Participants were not charged for their thyroid hormone status. The parents of all participants in the study signed an informed consent form. The information was recorded on the basis of honesty and utmost confidentiality.
Statistical analysis
Statistical data were entered into SPSS 21 software. Mean and standard deviation or frequency and percentage were used to display demographic information. Chi-square test was used to evaluate the relationship between body mass index and thyroid test status.
Findings
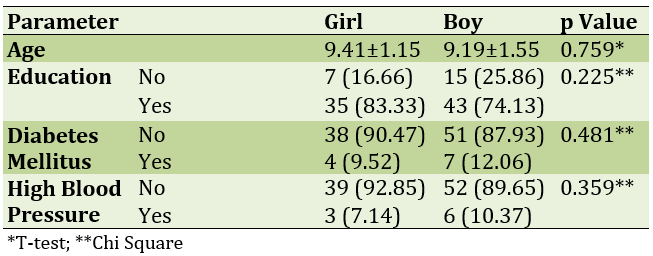
The mean age of study participants was 9.29±1.67 years; 42 of them were girls and 58 were boys. Twenty-two of them had not started their education and the rest had started their education. Eleven people had diabetes mellitus and nine had high blood pressure (Table 1).
Table 1) Comparison of demographic information of study participants by gender
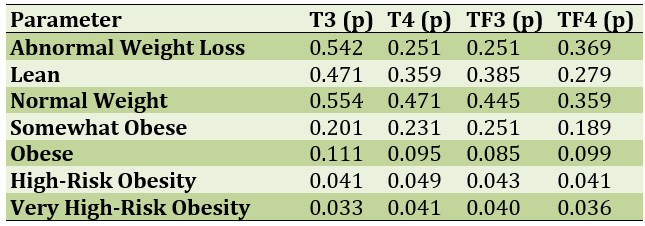
The children participating in the study were divided according to body mass index. They were also divided according to the amount of thyroid hormones. The results showed that the higher the body mass index, the lower the thyroid hormones; means that hypothyroidism leads to obesity in children (Table 2).
Table 2) Comparison of body mass index with thyroid hormones in study participants
Discussion
Deficiency and increased iodine intake both cause thyroid dysfunction. In moderate to severe iodine deficiency, mean serum TSH concentrations are often slightly higher than normal, while free T3 and T4 concentrations remain within the normal range [10]. As exacerbation of iodine deficiency, TSH increases further and goiter and clinical hypothyroidism may develop. On the other hand, increased iodine intake also leads to an increase in the incidence of mild hypothyroidism, often in patients with positive thyroid antibodies. Iodine-containing drugs such as amiodarone and its main metabolite called desethylamiodaron inhibit the enzyme iodothyronine de iodinase type 2, which converts T4 to T3, and are therefore known to increase TSH concentrations. In addition, attention has recently been paid to the role of iodine-enhancing iodine-induced radiographic contrast agents. In one study, children who underwent iodine-containing radiographs were 2.6 times more likely to develop hypothyroidism, although the timing and impact of such an outcome are still unclear [11]. In children treated with I131-metaiodobenzylguanidine for neuroblastoma, thyroid dysfunction occurs despite prophylactic administration of potassium iodide. The chances of developing this disorder increase over time, so in such children who survive treatment, screening for thyroid dysfunction on a regular basis is recommended. Coughs and iodine-containing supplements may also cause thyroid dysfunction [12].
Clinical guidelines of the American Thyroid Association and the European Thyroid Association for the administration of lutetroxin to adults with TSH>10mU/l, as well as those with TSH between 4.5 and 10mL/L with clinical signs of hypothyroidism or anti-thyroid antibody Have positive or evidence of atherosclerotic cardiovascular disease, recommend [13].
Treatment of subclinical hypothyroidism in children is still under discussion. In fact, the potential effects of mild hypothyroidism on the health outcomes of children are not well understood and the available evidence has not shown the effect of treatment on children's neurocognitive and physical development. Several studies have evaluated physical growth among children with autoimmune and non-autoimmune hypothyroidism, most of whom have shown normal physical growth even in children with long-term subclinical hypothyroidism [14].
In addition, no significant effects were observed on the growth of children treated with lutetroxine for 2 years due to clinical hypothyroidism. The available information on the effect of subclinical hypothyroidism on neurological outcomes in children is contradictory. Two small studies in children with subclinical hypothyroidism reported minor disturbances in the Attention process, but data from one large study showed normal cognitive performance in children with subclinical hypothyroidism. In addition, in a recent prospective case-control study among 30 children with idiopathic and long-term subclinical hypothyroidism in the control group with normal thyroid function, verbal performance processes and IQ levels were normal [9]. The two studies did not show any abnormalities in biochemical markers, bone metabolism, lumbar vertebral bone density, and bone quality in children with subclinical hypothyroidism [15, 16].
Recently, concerns about the impact of subclinical hypothyroidism on cardiovascular outcomes in patients with the disease have increased. Coronary heart disease and heart failure appear to be more common in adults with subclinical hypothyroidism, especially if the TSH level is greater than 10 milliunits per liter. At higher TSH concentrations, even in children, plasma lipid concentrations increase. In addition, recent case-control studies have shown that mild untreated hypothyroidism in children can be caused by factors responsible for premature cardiovascular complications such as dyslipidemia, increased visceral adipose tissue, increased hemocysteine concentration, and early detection of markers of dysfunction. Vascular endothelium and subclinical dysfunction of the left ventricle. Luteroxine treatment with beneficial effects on most biochemical markers has been responsible for cardiovascular complications and vascular endothelial function. However, despite the occurrence of such subclinical cardiovascular disorders, the available information is still insufficient to recommend treatment for subclinical hypothyroidism in all children. And treatment should be based on the individual condition of each patient [17, 18].
The first step in evaluating a child with subclinical hypothyroidism is to look at other causes of increased TSH, including laboratory error, periodic fluctuations in TSH secretion, and a transient increase after recovery from a non-thyroid or subacute thyroid disease. Persistent subclinical hypothyroidism should be demonstrated by re-measuring TSH after 4 to 12 weeks [19].
In children with persistent increases in TSH, diagnostic evaluation is necessary. In the history of the child, attention should be paid to the presence of neonatal hyperthyroidism, autoimmune or genetic conditions, the use of drugs that interfere with thyroid function, previous exposure to ionizing radiation, and endemic iodine deficiency. Attention to subclinical hypothyroidism, goiter, endocrine and autoimmune diseases in the affected child's family is also helpful. Clinical examination should consider the clinical signs and symptoms of hypothyroidism, goiter, weight gain, and clinical signs of specific genetic diseases. All patients should be screened for anti-thyroid antibodies [20].
Thyroid ultrasonography provides information about the morphology and structure of the thyroid gland. Measurement of urinary iodine concentration is recommended in children living in areas with endemic iodine deficiency. Measurement of serum lipids is necessary in children with subclinical hypothyroidism who are obese, skin pigment ectanthosis nigrican, and have a family history of dyslipidemia [21].
The patient's subsequent evaluation and decision on treatment depends on the etiology, the amount of TSH increase, the risk of progression to clinical hypothyroidism, and the presence of clinical signs and symptoms of mild hypothyroidism. In most cases, treatment with levothyroxine is indicated in cases where the child has autoimmune hypothyroidism, has a TSH>10mU/L, or has subclinical hypothyroidism with goiter or clinical signs. In children who do not have an indication in us, measurement of thyroid hormones every 6 months is recommended. Children with Turner and Down syndrome should be closely monitored because they are more likely to develop progressive thyroid dysfunction. Treatment of subclinical hypothyroidism in conjunction with other autoimmune diseases, including celiac disease or type 1 diabetes, is discussed. Although a recent study has shown that celiac disease in patients with subclinical hypothyroidism is a predictor of overt thyroid failure; However, there is still insufficient information on the effect of early treatment with levothyroxine in these children [22, 23].
TSH resistance should be considered in the differential diagnosis of all cases of non-autoimmune hypertrophy and goiter. The treatment of these cases is also questionable and depends on the age of the child and the severity of the increase in TSH. Treatment is necessary in cases of overt hypothyroidism, while close follow-up is recommended in mild and asymptomatic cases. In overweight or obese children, lifestyle changes and diet are recommended, and thyroid function is re-evaluated after weight loss. Iodine supplementation is prescribed in cases where the child lives in areas with endemic iodine deficiency or has low urinary iodine excretion. Luteiroxin is recommended in children taking thyroid-mediated drugs if TSH is> 10mU/L. Evaluation of thyroid function is necessary after stopping the responsible drug to ensure the return of normal thyroid function [24-26].
Conclusion
In all cases of subclinical hypothyroidism in children, following which thyroid function returns to normal, re-evaluation at older ages, especially during adolescence or pregnancy, is necessary.
Acknowledgements: None declared by the authors.
Ethical Permission: This study was conducted after approval by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.740).
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Most thyroid diseases seen in adults also occur in children. Although the treatment of these diseases in children is slightly different from adults, the principles in us are the same in both groups. Congenital hypothyroidism, congenital goiter, Hashimoto's thyroiditis and Graves' disease are the most important thyroid diseases in children [1, 2].
The normal growth and development of the fetal thyroid gland and the production of thyroid hormone are vital for the development of the brain during and after fetal life. The concentration of thyroid hormones in the fetal circulation is low in the first half of pregnancy. During this period, the fetus is completely dependent on maternal thyroid hormone, which passes through the placenta and reaches the fetus. The production of thyroid hormones (thyroxine and to a lesser extent triiodothyronine) in the fetal thyroid gland begins at 12 weeks of gestation [3, 4].
The prevalence of this disease varies in different countries and from one case in 3500 to 4000 live births have been reported. Studies in Iran have estimated this rate at one in 1000 live births. Thyroid hormone deficiency is associated with a wide range of neuropsychiatric disorders during the fetal life and the first year after birth. Congenital hypothyroidism can be primary (hypothyroidism), secondary (hypopharyngeal), and tertiary (hypothalamic) [5].
Congenital hypothyroidism can be transient or permanent. The primary and permanent type is the most common type of congenital hypothyroidism in infants. About 80 to 90 percent of the cause of permanent congenital hypothyroidism is monocytoma and is due to an evolutionary disorder of the thyroid gland, meaning that the thyroid gland may not be present at all or may be smaller than normal. Abnormal growth of the thyroid gland is also a type of developmental defect [6]. The ectopic thyroid gland may be located anywhere from behind the tongue to its normal location in the front of the neck. 10 to 20% of cases are due to problems in the production of thyroid hormones [7]. These disorders are inherited and are more common in consanguineous marriages. Permanent hypothyroidism of central origin (disorder of the hypothalamus or hypophysis) is very rare and usually affects one in 50,000 infants. This type of congenital hypothyroidism is always accompanied by a deficiency of other hormones in the pituitary gland or the hypo to the mouse. Transient types of congenital hypothyroidism are prevalent in areas with moderate to severe iodine deficiency [8].
Only a handful of infants with congenital hypothyroidism show clinical signs and symptoms of hypothyroidism at birth, including delayed birth, macular degeneration, jaundice, and delayed skeletal system development. A small percentage of infants have goiter. Other signs and symptoms include malnutrition, hypothermia, constipation, prolonged jaundice, abdominal distention, umbilical hernia, dry skin, large tongue, and heavy crying [6].
Because most babies with congenital hypothyroidism do not show signs and symptoms of hypothyroidism at birth, a screening program is recommended to diagnose the disease. Today, in most countries of the world, including Iran, a screening program is performed by taking a blood sample 2 to 5 days after birth from the heel. In this blood sample, the levels of TSH and T4 are measured. If they are abnormal, the baby will be treated with levothyroxine tablets. Early diagnosis and early treatment prevent the baby from developing mental retardation and promote normal growth [9].
In some studies, obesity has been linked to pediatric thyroid disorders; This relationship can lead to many risks in children; Because both of these diseases have side effects that can occur more severely together; High blood pressure, disorders of the immune system, as well as cardiovascular disease occur in children who are obese and have thyroid disorders 16 times more often than normal children; The aim of this study was the relationship between hypothyroidism and body mass index in children.
Instrument and Methods
Study design
This is a cross-sectional descriptive study that was conducted during 2019 in hospital centers affiliated to Tabriz University of Medical Sciences with the participation of 100 people under 12 years old. The sampling method was available and available among children referred to the clinics of hospitals in Tabriz. All participants were assessed based on inclusion and exclusion criteria.
Inclusion criteria included age under 12, consent to participate in the study, ability to participate in the study, and comfort in performing the relevant tests; Exclusion criteria included vitamin D intake, calcium intake, intake of any multivitamin, musculoskeletal disorders and cancers, and receiving chemotherapy drugs.
Procedure
All participants in the study were examined for demographic factors. These factors included age, gender, education, history of diabetes mellitus, and history of uncontrolled hypertension. Then, with the least amount of clothing, their weight was measured with the help of a scale (with an accuracy of half a kilogram) made in Germany (Seca). After that, their height was measured with the help of a meter with an accuracy of half a centimeter. All participants were leaning against the wall, standing upright on the floor, and their height was measured. Then their body mass index was measured. Thyroid tests were then taken from all participants on an empty stomach. The fasting thyroid test is done by taking a blood sample in a blood laboratory, and this test is done for the baby through the baby's heel. In a thyroid test, the amount of T4, T3, and TSH is checked to determine the type of thyroid of the affected person, whether the thyroid is overactive or underactive. In general, thyroid testing does not require special preparation and work, only fasting is important in thyroid testing. It should be noted that eating my job, peanuts, and cabbage, which cause iodine excretion, were banned for three days before the test. The children were then exposed to both normal and abnormal levels based on total thyroxine (T4), free thyroxine (TF4), complete triiodothyronine (T3) and free triiodothyronine. Children's body mass index was also categorized as: lean/abnormal weight loss, lean, normal weight, somewhat obese, obese, high-risk obesity, and very high-risk obesity/abnormal obesity.
Ethical considerations
Participants were not charged for their thyroid hormone status. The parents of all participants in the study signed an informed consent form. The information was recorded on the basis of honesty and utmost confidentiality.
Statistical analysis
Statistical data were entered into SPSS 21 software. Mean and standard deviation or frequency and percentage were used to display demographic information. Chi-square test was used to evaluate the relationship between body mass index and thyroid test status.
Findings
The mean age of study participants was 9.29±1.67 years; 42 of them were girls and 58 were boys. Twenty-two of them had not started their education and the rest had started their education. Eleven people had diabetes mellitus and nine had high blood pressure (Table 1).
Table 1) Comparison of demographic information of study participants by gender

The children participating in the study were divided according to body mass index. They were also divided according to the amount of thyroid hormones. The results showed that the higher the body mass index, the lower the thyroid hormones; means that hypothyroidism leads to obesity in children (Table 2).
Table 2) Comparison of body mass index with thyroid hormones in study participants

Discussion
Deficiency and increased iodine intake both cause thyroid dysfunction. In moderate to severe iodine deficiency, mean serum TSH concentrations are often slightly higher than normal, while free T3 and T4 concentrations remain within the normal range [10]. As exacerbation of iodine deficiency, TSH increases further and goiter and clinical hypothyroidism may develop. On the other hand, increased iodine intake also leads to an increase in the incidence of mild hypothyroidism, often in patients with positive thyroid antibodies. Iodine-containing drugs such as amiodarone and its main metabolite called desethylamiodaron inhibit the enzyme iodothyronine de iodinase type 2, which converts T4 to T3, and are therefore known to increase TSH concentrations. In addition, attention has recently been paid to the role of iodine-enhancing iodine-induced radiographic contrast agents. In one study, children who underwent iodine-containing radiographs were 2.6 times more likely to develop hypothyroidism, although the timing and impact of such an outcome are still unclear [11]. In children treated with I131-metaiodobenzylguanidine for neuroblastoma, thyroid dysfunction occurs despite prophylactic administration of potassium iodide. The chances of developing this disorder increase over time, so in such children who survive treatment, screening for thyroid dysfunction on a regular basis is recommended. Coughs and iodine-containing supplements may also cause thyroid dysfunction [12].
Clinical guidelines of the American Thyroid Association and the European Thyroid Association for the administration of lutetroxin to adults with TSH>10mU/l, as well as those with TSH between 4.5 and 10mL/L with clinical signs of hypothyroidism or anti-thyroid antibody Have positive or evidence of atherosclerotic cardiovascular disease, recommend [13].
Treatment of subclinical hypothyroidism in children is still under discussion. In fact, the potential effects of mild hypothyroidism on the health outcomes of children are not well understood and the available evidence has not shown the effect of treatment on children's neurocognitive and physical development. Several studies have evaluated physical growth among children with autoimmune and non-autoimmune hypothyroidism, most of whom have shown normal physical growth even in children with long-term subclinical hypothyroidism [14].
In addition, no significant effects were observed on the growth of children treated with lutetroxine for 2 years due to clinical hypothyroidism. The available information on the effect of subclinical hypothyroidism on neurological outcomes in children is contradictory. Two small studies in children with subclinical hypothyroidism reported minor disturbances in the Attention process, but data from one large study showed normal cognitive performance in children with subclinical hypothyroidism. In addition, in a recent prospective case-control study among 30 children with idiopathic and long-term subclinical hypothyroidism in the control group with normal thyroid function, verbal performance processes and IQ levels were normal [9]. The two studies did not show any abnormalities in biochemical markers, bone metabolism, lumbar vertebral bone density, and bone quality in children with subclinical hypothyroidism [15, 16].
Recently, concerns about the impact of subclinical hypothyroidism on cardiovascular outcomes in patients with the disease have increased. Coronary heart disease and heart failure appear to be more common in adults with subclinical hypothyroidism, especially if the TSH level is greater than 10 milliunits per liter. At higher TSH concentrations, even in children, plasma lipid concentrations increase. In addition, recent case-control studies have shown that mild untreated hypothyroidism in children can be caused by factors responsible for premature cardiovascular complications such as dyslipidemia, increased visceral adipose tissue, increased hemocysteine concentration, and early detection of markers of dysfunction. Vascular endothelium and subclinical dysfunction of the left ventricle. Luteroxine treatment with beneficial effects on most biochemical markers has been responsible for cardiovascular complications and vascular endothelial function. However, despite the occurrence of such subclinical cardiovascular disorders, the available information is still insufficient to recommend treatment for subclinical hypothyroidism in all children. And treatment should be based on the individual condition of each patient [17, 18].
The first step in evaluating a child with subclinical hypothyroidism is to look at other causes of increased TSH, including laboratory error, periodic fluctuations in TSH secretion, and a transient increase after recovery from a non-thyroid or subacute thyroid disease. Persistent subclinical hypothyroidism should be demonstrated by re-measuring TSH after 4 to 12 weeks [19].
In children with persistent increases in TSH, diagnostic evaluation is necessary. In the history of the child, attention should be paid to the presence of neonatal hyperthyroidism, autoimmune or genetic conditions, the use of drugs that interfere with thyroid function, previous exposure to ionizing radiation, and endemic iodine deficiency. Attention to subclinical hypothyroidism, goiter, endocrine and autoimmune diseases in the affected child's family is also helpful. Clinical examination should consider the clinical signs and symptoms of hypothyroidism, goiter, weight gain, and clinical signs of specific genetic diseases. All patients should be screened for anti-thyroid antibodies [20].
Thyroid ultrasonography provides information about the morphology and structure of the thyroid gland. Measurement of urinary iodine concentration is recommended in children living in areas with endemic iodine deficiency. Measurement of serum lipids is necessary in children with subclinical hypothyroidism who are obese, skin pigment ectanthosis nigrican, and have a family history of dyslipidemia [21].
The patient's subsequent evaluation and decision on treatment depends on the etiology, the amount of TSH increase, the risk of progression to clinical hypothyroidism, and the presence of clinical signs and symptoms of mild hypothyroidism. In most cases, treatment with levothyroxine is indicated in cases where the child has autoimmune hypothyroidism, has a TSH>10mU/L, or has subclinical hypothyroidism with goiter or clinical signs. In children who do not have an indication in us, measurement of thyroid hormones every 6 months is recommended. Children with Turner and Down syndrome should be closely monitored because they are more likely to develop progressive thyroid dysfunction. Treatment of subclinical hypothyroidism in conjunction with other autoimmune diseases, including celiac disease or type 1 diabetes, is discussed. Although a recent study has shown that celiac disease in patients with subclinical hypothyroidism is a predictor of overt thyroid failure; However, there is still insufficient information on the effect of early treatment with levothyroxine in these children [22, 23].
TSH resistance should be considered in the differential diagnosis of all cases of non-autoimmune hypertrophy and goiter. The treatment of these cases is also questionable and depends on the age of the child and the severity of the increase in TSH. Treatment is necessary in cases of overt hypothyroidism, while close follow-up is recommended in mild and asymptomatic cases. In overweight or obese children, lifestyle changes and diet are recommended, and thyroid function is re-evaluated after weight loss. Iodine supplementation is prescribed in cases where the child lives in areas with endemic iodine deficiency or has low urinary iodine excretion. Luteiroxin is recommended in children taking thyroid-mediated drugs if TSH is> 10mU/L. Evaluation of thyroid function is necessary after stopping the responsible drug to ensure the return of normal thyroid function [24-26].
Conclusion
In all cases of subclinical hypothyroidism in children, following which thyroid function returns to normal, re-evaluation at older ages, especially during adolescence or pregnancy, is necessary.
Acknowledgements: None declared by the authors.
Ethical Permission: This study was conducted after approval by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1398.740).
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Rumińska M, Witkowska-Sędek E, Majcher A, Pyrżak B. Thyroid function in obese children and adolescents and its association with anthropometric and metabolic parameters. Adv Exp Med Boil. 2016;912:33-41. [Link] [DOI:10.1007/5584_2016_232]
2. Unüvar T, Anık A, Catlı G, Esen I, Abacı A, Büyükgebiz A, et al. Isolated hyperthyrotropinemia in childhood obesity and its relation with metabolic parameters. J Endocrinol Invest. 2014;37(9):799-804. [Link] [DOI:10.1007/s40618-014-0100-y]
3. Dahl M, Ohrt JD, Fonvig CE, Kloppenborg JT, Pedersen O, Hansen T, et al. Subclinical hypothyroidism in danish lean and obese children and adolescents. J Clin Res Pediatr Endocrinol. 2017;9(1):8-16. [Link] [DOI:10.4274/jcrpe.3319]
4. Ghergherehchi R, Hazhir N. Thyroid hormonal status among children with obesity. Ther Adv Endocrinol Metab. 2015;6(2):51-5. [Link] [DOI:10.1177/2042018815571892]
5. Damiano F, Rochira A, Gnoni A, Siculella L. Action of thyroid hormones, t3 and t2, on hepatic fatty acids: differences in metabolic effects and molecular mechanisms. Int J Mol Sci. 2017;18(4):744. [Link] [DOI:10.3390/ijms18040744]
6. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based Med. 2015;8(1):2-10. [Link] [DOI:10.1111/jebm.12141]
7. Peeters RP, Engl N. Subclinical hypothyroidism. Mass Med Soc. 2017;376:2556-65. [Link] [DOI:10.1056/NEJMcp1611144]
8. Sun X, Sun Y, Li WC, Chen CY, Chiu YH, Chien HY, et al. Association of thyroid-stimulating hormone and cardiovascular risk factors. Intern Med. 2015;54(20):2537-44. [Link] [DOI:10.2169/internalmedicine.54.4514]
9. Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric obesity-assessment, treatment, and prevention: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(3):709-57. [Link] [DOI:10.1210/jc.2016-2573]
10. Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. measurement of blood pressure in humans: a scientific statement from the American heart association. Hypertension 2019;73(5):e35-66. [Link] [DOI:10.1161/HYP.0000000000000087]
11. Guo Z, Li M, Han B, Qi X. Association of non-alcoholic fatty liver disease with thyroid function: A systematic review and meta-analysis. Dig Liver Dis. 2018;50(11):1153-62. [Link] [DOI:10.1016/j.dld.2018.08.012]
12. Kaltenbach TE, Graeter T, Oeztuerk S, Holzner D, Kratzer W, Wabitsch M, et al. Thyroid dysfunction and hepatic steatosis in overweight children and adolescents. Pediatr Obes. 2017;12(1):67-74. [Link] [DOI:10.1111/ijpo.12110]
13. Rumińska M, Witkowska-Sędek E, Majcher A, Pyrżak B. Thyroid function in obese children and adolescents and its association with anthropometric and metabolic parameters. Adv Exp Med Biol. 2016;912:33-41. [Link] [DOI:10.1007/5584_2016_232]
14. Licenziati MR, Valerio G, Vetrani I, De Maria G, Liotta F, Radetti G. Altered Thyroid function and structure in children and adolescents who are overweight and obese: reversal after weight loss. J Clin Endocrinol Metab. 2019;104(11):2757-65. [Link] [DOI:10.1210/jc.2018-02399]
15. Saboktakin L, Bilan N, Ghalehgolab Behbahan A, Poorebrahim S. Relationship between resistin levels and sepsis among children under 12 years of age: A case control study. Front Pediatr. 2019;7:355. [Link] [DOI:10.3389/fped.2019.00355]
16. Krause AJ, Cines B, Pogrebniak E, Sherafat‐Kazemzadeh R, Demidowich AP, Galescu OA, et al. Associations between adiposity and indicators of thyroid status in children and adolescents. Pediatr Obes. 2016;11(6):551-8. [Link] [DOI:10.1111/ijpo.12112]
17. Dahl M, Ohrt JD, Fonvig CE, Fonvig CE, Kloppenborg JT, Pedersen O, et al. Subclinical hypothyroidism in danish lean and obese children and adolescents. J Clin Res Pediatr Endocrinol. 2017;9(1):8-16. [Link] [DOI:10.4274/jcrpe.3319]
18. Thiagarajan S, Arun Babu T, Balaji R. Subclinical hypothyroidism in obese south Indian children. Indian J Pediatr. 2019;86(7):662. [Link] [DOI:10.1007/s12098-019-02966-9]
19. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;9:521. [Link] [DOI:10.3389/fimmu.2017.00521]
20. Singh R. Does one size fit everyone? replacement dose of levothyroxine in long-standing primary hypothyroidism in adults. Indian J Endocrinol Metab. 2017;21(3):404-9. [Link] [DOI:10.4103/ijem.IJEM_502_16]
21. Duntas LH, Jonklaas J. Levothyroxine dose adjustment to optimise therapy throughout a patient's lifetime. Adv Ther. 2019;36(2):30-46. [Link] [DOI:10.1007/s12325-019-01078-2]
22. Küme T, Acar S, Tuhan H, Çatlı G, Anık A, Gürsoy Çalan O, Böber E, et al. The relationship between serum zonulin level and clinical and laboratory parameters of childhood obesity. J Clin Res Pediatr Endocrinol. 2017;9(1):31-8. [Link] [DOI:10.4274/jcrpe.3682]
23. Khaleghi S, Ju JM, Lamba A, Murray JA. The potential utility of tight junction regulation in celiac disease: focus on larazotide acetate. Therap Adv Gastroenterol. 2016;9(1):37-49. [Link] [DOI:10.1177/1756283X15616576]
24. Sturgeon C, Lan J, Fasano A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann N Y Acad Sci. 2017;1397(1):130-42. [Link] [DOI:10.1111/nyas.13343]
25. Virili C, Fallahi P, Antonelli A, Benvenga S, Centanni M. Gut microbiota and Hashimoto's thyroiditis. Endocr Metab Disord. 2018;19(4):293-300. [Link] [DOI:10.1007/s11154-018-9467-y]
26. Virili C, Centanni M. Does microbiota composition affect thyroid homeostasis?. Endocrine. 2015;49(3):583-7. [Link] [DOI:10.1007/s12020-014-0509-2]









