GMJ Medicine
eISSN : 2626-3041
Volume 2, Issue 4 (2023)
GMJM 2023, 2(4): 109-114 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/03/7 | Accepted: 2023/09/14 | Published: 2023/10/25
Received: 2023/03/7 | Accepted: 2023/09/14 | Published: 2023/10/25
How to cite this article
Sharifi S, Saberi K, Rahmanian M, Bakhshandeh A. Effect of Perioperative Administration of Dexmedetomidine in Cardiac Surgeries. GMJM 2023; 2 (4) :109-114
URL: http://gmedicine.de/article-2-213-en.html
URL: http://gmedicine.de/article-2-213-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- National Nutrition and Food Technology Research Institute, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2- Imam Khomeini Medical and Research Centre, Tehran University of Medical Sciences, Tehran, Iran
2- Imam Khomeini Medical and Research Centre, Tehran University of Medical Sciences, Tehran, Iran
Keywords:
| Abstract (HTML) (1785 Views)
Full-Text: (697 Views)
Introduction
Before introduction of coronary artery bypass grafting (CABG), a reliable method for healing coronary stenosis, most of the patients must undergo cardiac arrest using a cardiopulmonary pump (CPB); the traditional approach of using CPB gave its place to the newer off-pump coronary artery bypass grafting with respect to fixation technology [1]. Despite these and many other developments, yet 5%–30% of patients undergoing cardiac surgeries may become challenged due to renal dysfunction [2], delirium [3, 4], pathological changes in electrocardiogram (EKG) rhythms [5]. Eliminating or reducing these unwanted outcomes is likely to improve the prognosis in patients who have undergone cardiac surgery. Therefore, various approaches, tools, and drugs have been clinically applied to reduce the incidence of post-operative issues.
Several studies, consisting of laboratory and clinical researches, suggested that dexmedetomidine (DEX) may be useful for reducing the adverse effect of cardiac surgeries [6, 7]. Studies indicated that DEX is associated with fewer incidences of post-operative complications in patients undergoing cardiac surgery. Current guidelines suggest using either propofol or DEX instead of benzodiazepines to improve clinical outcomes [3].
DEX is a highly selective, short-acting α-2 adrenergic agonist that initially has been used as an intravenous anti-delirium drug in ICU and then approved as a sedative [8]. Besides safe and effective sedation, it may significantly reduce the use of analgesics, β-blockers, anti-emetics, epinephrine, and diuretics. Recent studies also found that DEX has a protective effect on the kidney [7].
Kidney injury can lead to renal insufficiency, hyperkalemia, water intoxication, fatal arrhythmia, and cerebral edema, which are often life-threatening. DEX decreases the norepinephrine level in the blood, and thus it induces renal artery vasodilatation and increases renal blood flow and urine output [9].
The purpose of the current study was to investigate the outcome of administration of DEX in patients undergoing cardiac surgeries from different aspects.
Materials and Methods
In this retrospective study, all patients who meet our criteria at Imam Khomeini Hospital, from August to November 2018 entered our study. The sample size was estimated using SPSS sample power (release 3.0.1), 17 patients were sufficient for each group to achieve a power of 80% considering type one error of less than 5%.
Anyone who was a candidate for CABG without a valve, CABG with valve surgery, and valve-only
surgery was entered the study. We did not enter the patients with any aorta complication or any surgery that needed deep hypothermic circulatory arrest. A total of 72 patients entered the study, in which 21 cases were excluded. Our exclusion criteria were any urological or nephrological problem consists of: a base creatinine more than 2, history of permanent or temporary dialysis, hydronephrosis or kidney infection, history of contrast administration for MRI or CT-scan, urine retention and using diapers or Foley catheter and any renal surgery such as nephrectomy or transplant; Also, we excluded the patients with history of long-term sedative drugs (such as oxazepam, diazepam, clonazepam, alprazolam, gabapentin, etc.), history of anti-depressant use (such as sertraline, fluoxetine, fluvoxamine, etc.), any drug abuse, exciter drugs usage (like crystal, ritalin, cocaine, etc.), any sleep disorder detected in tests and non-invasive ventilator extended usage. A total of 51 patients entered our study of which 31% of them were in the DEX group, and 18% were in control group who double-blindly received whether DEX infusion of normal saline 0.9%, and then data were extracted retrospectively according to patients’ file; neither the clinical staffs (consist of anesthesia crew, surgical crew and nursing group), nor data analyzer were aware of the groups until the end of analyze.
We infused DEX, 10min before anesthesia induction in a 0.2–0.6µg/Kg/h. An arterial-line was then fixed in the radial or brachial site. After standard monitoring (consists of a 5-lead EKG, pulse oximetry, and non-invasive blood pressure), the induction of anesthesia was performed using propofol, fentanyl, midazolam, atracurium. Anesthesia was then maintained using an infusion of midazolam and propofol. A routine ABG was sent before induction, 5min after induction, before cross-clamp, during the pump as needed and after disconnecting from the pump and before transferring to ICU. ABG was assessed, and improvement of ventilator tidal volume and respiratory rate was adjusted as needed along with drug administration if needed. An end-tidal CO2 of 35–45 was considered as normal.
DEX infusion was continued post-operatively until weaning from the ventilator. If the patient was not extubated after 24h, it was discontinued due to FDA recommendations on safe administration of DEX. If any drug needed infusion during and after the surgery, the data were documented. The data for hemodynamic (HR, systolic, and diastolic pressure) were documented during and after the surgery, according to the standard sheet. Mean arterial pressure (MAP) was then calculated using the formula and compared before induction and average of post-operative data. Richmond agitation-sedation scale (RASS; Table 1) and critical-care pain observation tool (CPOT; Table 2) was assessed.
Table 1) Richmond Agitation-Sedation Scale

Table 2) The Critical-Care Pain Observation Tool (CPOT)
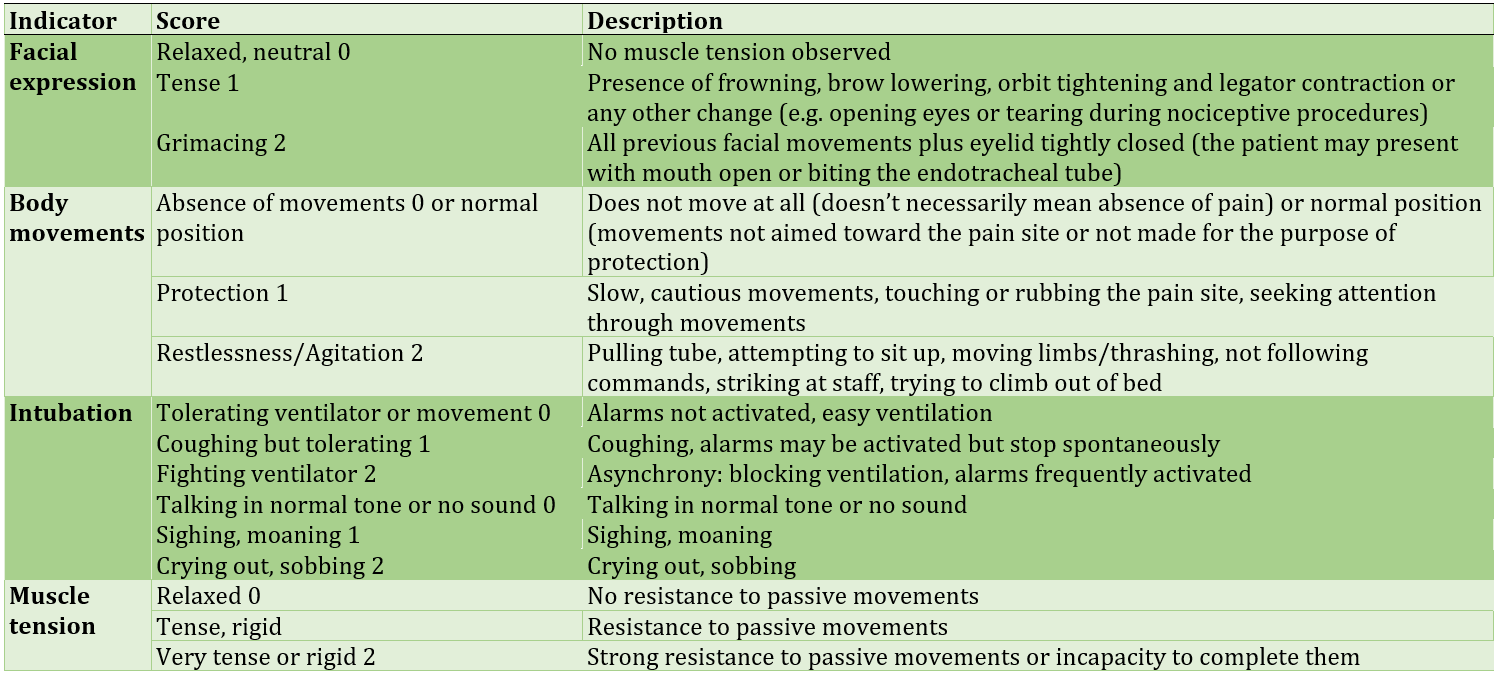
Table 3) The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)
Daily routine experiments for all the patients were BUN, creatinine, hematocrit, hemoglobin, platelets count, and WBC. The initial goal of the study was to detect atrial fibrillation (AF) rhythm from EKG interpretation, Acute Kidney Injury (AKI), according to the latest KDIGO guidelines, delirium assessed by the Confusion Assessment Method for Intensive Care Unit (CAM-ICU), mechanical ventilation by minutes and days of ICU stay after surgery (Table 3).
Data were analyzed using SPSS 25 software and expressed as mean (lower bond–upper bond confidence interval 95%) for continuous variables and number (percentage) for categorical variables. Data were then analyzed using one-way ANOVA for continuous data and Chi-square for categorical data.
Findings
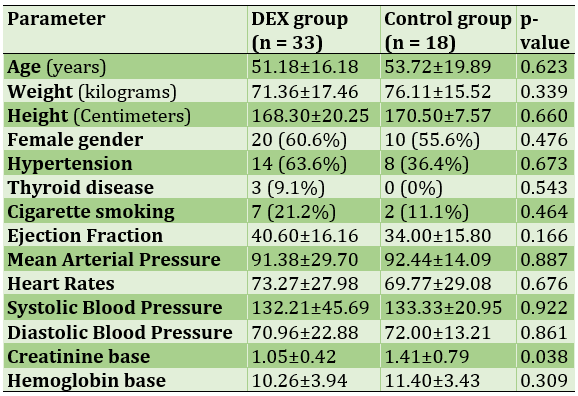
We had a total of 51 persons, 33 (64.70%) in the DEX group and 18 (35.29%) in the control group (Table 4).
Table 4) Patients’ characteristics
From the interpretation of the 12 lead EKG of the patients before the surgery, 3 (5.9%) had an AF rhythm of which 2 (66.7%) were in the DEX group and 1 (33.3%) was in the control group (p>0.05). No bradycardia was reported. Only 1 (3%) of the DEX patients had more than 140 beats per minute tachycardia, and no one in the control group had presented with tachycardia (p>0.05). Neither PAC nor PVC rhythm was detected in this research. Also, EKGs indicated no flutter, myocardial infarction, and ischemic heart disease. 28 (63.6%) of the patients with normal cardiac rhythm were in the DEX group, and 16 (36.4%) of them were in our control group (p>0.05).
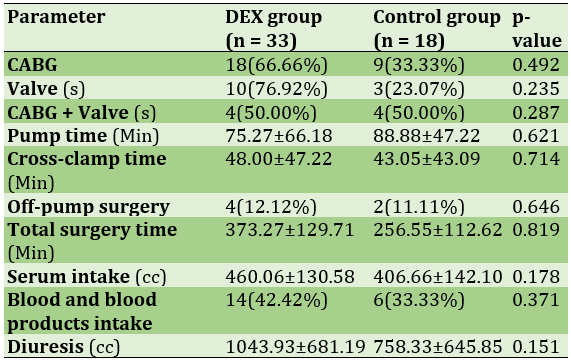
A total of 27 (52.9%) of the patients had only done CABG surgery, 18 (54.5%) from the DEX group and 9 (50.0%) from the control group (p>0.05; Table 5).
During the surgery, a total of 20 (39.2%) patients required blood products (packed cell, fresh frozen plasma, platelets); 14 (42.4%) of the DEX patients and 6 (33.3%) of the control group (p>0.05). 8 (24.2%) of the DEX group and 3 (16.7%) of the control group needed electroshock due to surgeons’ demand (p>0.05). In the routine ABGs, we detected 2 (6.1%) acidosis in the DEX group and 1 (5.6%) in the control group (p>0.05) from which all three of them needed improvement with sodium bicarbonate (p>0.05) and all of them solved (p>0.05).
Table 5) Perioperative data are described as Mean±Standard deviation or Number (percentage within group)
A total of 25 (49.0%) of the patients needed an infusion of epinephrine, which consists of 14 (42.4%) of the DEX group and 11 (61.1%) of the control group (p>0.05). Also, one patient from each group (3.0% of DEX and 5.6% of the control group) needed norepinephrine (p>0.05). 28 (84.8%) of the DEX group and 17 (94.4%) of the control group received midazolam infusion (p>0.05). Also, 28 (84.8%) of the DEX patients and 16 (88.9%) of the control group sedated with fentanyl drip (p>0.05). Only 2 (6.1%) of the DEX patients needed dopamine, and none of the control group needed (p>0.05). No patient received milrinone. 16 (48.5%) of the DEX group and 11 (40.7%) of the control group required TNG due to hypertension (p>0.05). One of the DEX (3.0%) and one of the control group (5.6%) received furosemide infusion (p>0.05). Mean MAP before surgery for the DEX group was 91.38 (80.84–101.91 CI 95%), and for the control group was 92.44 (85.43–99.45 CI 95%) (p>0.05). After the surgery, during the stay in ICU, the mean MAP was 61.12 (55.43–66.81 CI 95%) in the DEX group and 65.50 (56.23–74.77 CI 95%) in control group (p>0.05).
The mean total mechanical ventilation time was 589.36 (527.66–651.06 CI 95%) minutes in the DEX group and 521.11 (409.50–632.71 CI 95%) minutes in the control group (p>0.05). Total ICU stays in the day have a mean of 2.36 days in the DEX group and 2.77 days in the control group (p>0.05). From our end points, six patients came front with AF ehythm of which only one (16.7%) of them were from the DEX group and the other 5 (83.3%) were from the control group (p=0.009). No patient required dialysis after the surgery. No patient underwent redo surgery, and no one faced delirium. Seven patients seemed to be AKI, which five (71.4%) were in the DEX group, and two (28.6%) were in the control group (p>0.05).
Discussion
This research was designed to investigate the effect of DEX on patients undergoing cardiac surgery. The results did not show any significant difference between the group who received DEX and the control group for AKI; indeed, we had a total of seven AKI patients, which according to KDIGO classification, two of them were stage 2 and no patient from current study became stage 3 AKI. There is a different possibility for this result; first that in our study, we used different drugs, especially midazolam and fentanyl, along with DEX which may affect the renoprotective characteristic of DEX.7 In a study from Ammar et al., creatinine increased significantly higher in the DEX group on day 1. Also, they reported DEX might provide cardiac and renal protection during cardiac surgery though it had no impact on postoperative outcomes [1, 8, 10]. However, some studies suggested that it may have a favorable impact on outcomes in patients with pre-existing cardiac and/or renal dysfunction [11]. In our study, we had excluded patients with any renal complication, and more studies on those patients are recommended. The results contradicted that of Liu et al. whereas they concluded perioperative administration of DEX in adult patients undergoing cardiac surgery might reduce the incidence of post-operative AKI [12]. Future trials are needed to reduce the contradiction in nephrological effects of DEX in cardiac surgery patients.
Another reason that increases the rate of AKI is low blood pressure and/or unstable hemodynamics. The etiology of renal injury is due mainly to the elevation of renin levels as a result of sympathetic overactivity in addition to nephrotoxic, inflammatory, and hemodynamic components. The patients in our study had a mean MAP of 91.38 for the DEX group and 92.44 for the control group before surgery and 61.12 for the DEX group and 65.50 for the control group after surgery. The results were not statistically significant, which was in line with Chang et al. study [13]. Despite this fact, we see a lower MAP in the DEX group, which may explain why we had more AKI in the DEX than in the control group.
Despite diminution in blood pressure, the pain-relieving characteristics of DEX are questionable. In a study about fast-track management in off-pump CABG from Zientara et al., the DEX group needed less use of pain medication in the initial phase at ICU [8]. In the current research, 84.8% of the DEX group and 88.9% of the control group required fentanyl infusion. These results revealed that most of the patients with or without DEX might need another pain-reliever. In our study, we chose to use an infusion of fentanyl instead of any drug stat administration, because the strategy in controlling pain is to prevent it beforehand, instead of healing it.
In contrary to DEX low-potency in relieving pain, it can significantly increase the time of weaning. Mean mechanical ventilation time for the DEX group was 68 min higher compare to the control group; this was contrary to some other studies [8, 14], whereas they used propofol infusion for their control group. Although we used a placebo instead of propofol or any other drugs, for reaching a RASS about -1 to -2, we need to perfuse more of midazolam and fentanyl in the control group. This might explain why, in some cases, DEX group wanted more time for weaning from ventilator. Although DEX has effects on the brain locus coeruleus and the a2-adrenergic receptors of the spinal cord to result in sedation, sympatholytic, analgesia, and anti-nociception, both groups had the same CPOT and RASS in our study. This was in line with some other studies [15]. Besides, DEX did not show any benefit in deep sedation [16], and because of that, we should only consider it as an adjuvant to other drugs. The current research did not show any difference between groups for pain. This was contrary to some studies; among them a meta-analysis from Wang et al. reported that DEX could effectively relieve the pain intensity, extend the pain-free period, and decrease the consumption of opioids during post-operative recovery of adults in general anesthesia [17]; however, their study extracted the data only from one cardiac surgery article. Further, another study in non-cardiac ICU suggests the advantage of DEX in analgesia [18]. Indeed, we recommend a study explicitly designed for assessment of DEX capability to relieve pain in cardiac surgery patients; because the power of sample of our study was according to AKI-occurrence, the results cannot be trusted alone. All the patients experienced a mean ICU stay of about 2 days, and there was no difference between groups from that aspect of view.
The results indicated a meaningful difference between groups for a new-onset AF rhythm after the surgery. This was in line with some other studies [5, 8] and explain the anti-arrhythmic characteristic of DEX [19]. However, a meta-analysis by Zhu et al [20]. revealed that DEX could not reduce the incidence of AF compared to control medicines following cardiac surgery. DEX might have an increased influence on AF occurrence if patients had a history of AF. We had three patients in our study, which had AF rhythm before surgery, but none of them had AF rhythm after surgery.
Most studies suggest that the post-operative administration of DEX may reduce delirium in patients, particularly following cardiac surgery [2, 4, 6, 21]. We used the CAM-ICU tool to screen for delirium based on four features: (1) a fluctuating mental status, (2) inattention, (3) disorganized thinking, and (4) altered level of consciousness. For the determination of delirium, a patient must display features (1) and (2), with either (3) or (4). In the current study, patients had not delirium. This may be a result of having a mean RASS of -2, which indicated a good and deep sleep. Although DEX known to improve the quality of sleep in critically ill patients [3, 22], reduces agitation [23] and reduces pain after cardiothoracic surgery [24, 25], we did not see any difference of RASS and CPOT between groups. This is probably due to the fact that patients from both groups received midazolam and fentanyl as needed. Further studies on DEX anti-arrhythmic effects is suggested.
Conclusion
There might be a meaningful reduction of new-onset AF rhythm in adult patients who use DEX after cardiac surgeries.
Acknowledgements: None declared by the authors.
Ethical Permission: The need for clinical trial registration was waived by the Ethics Committee of the Tehran University of Medical Sciences due to lack of clinical intervention.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Before introduction of coronary artery bypass grafting (CABG), a reliable method for healing coronary stenosis, most of the patients must undergo cardiac arrest using a cardiopulmonary pump (CPB); the traditional approach of using CPB gave its place to the newer off-pump coronary artery bypass grafting with respect to fixation technology [1]. Despite these and many other developments, yet 5%–30% of patients undergoing cardiac surgeries may become challenged due to renal dysfunction [2], delirium [3, 4], pathological changes in electrocardiogram (EKG) rhythms [5]. Eliminating or reducing these unwanted outcomes is likely to improve the prognosis in patients who have undergone cardiac surgery. Therefore, various approaches, tools, and drugs have been clinically applied to reduce the incidence of post-operative issues.
Several studies, consisting of laboratory and clinical researches, suggested that dexmedetomidine (DEX) may be useful for reducing the adverse effect of cardiac surgeries [6, 7]. Studies indicated that DEX is associated with fewer incidences of post-operative complications in patients undergoing cardiac surgery. Current guidelines suggest using either propofol or DEX instead of benzodiazepines to improve clinical outcomes [3].
DEX is a highly selective, short-acting α-2 adrenergic agonist that initially has been used as an intravenous anti-delirium drug in ICU and then approved as a sedative [8]. Besides safe and effective sedation, it may significantly reduce the use of analgesics, β-blockers, anti-emetics, epinephrine, and diuretics. Recent studies also found that DEX has a protective effect on the kidney [7].
Kidney injury can lead to renal insufficiency, hyperkalemia, water intoxication, fatal arrhythmia, and cerebral edema, which are often life-threatening. DEX decreases the norepinephrine level in the blood, and thus it induces renal artery vasodilatation and increases renal blood flow and urine output [9].
The purpose of the current study was to investigate the outcome of administration of DEX in patients undergoing cardiac surgeries from different aspects.
Materials and Methods
In this retrospective study, all patients who meet our criteria at Imam Khomeini Hospital, from August to November 2018 entered our study. The sample size was estimated using SPSS sample power (release 3.0.1), 17 patients were sufficient for each group to achieve a power of 80% considering type one error of less than 5%.
Anyone who was a candidate for CABG without a valve, CABG with valve surgery, and valve-only
surgery was entered the study. We did not enter the patients with any aorta complication or any surgery that needed deep hypothermic circulatory arrest. A total of 72 patients entered the study, in which 21 cases were excluded. Our exclusion criteria were any urological or nephrological problem consists of: a base creatinine more than 2, history of permanent or temporary dialysis, hydronephrosis or kidney infection, history of contrast administration for MRI or CT-scan, urine retention and using diapers or Foley catheter and any renal surgery such as nephrectomy or transplant; Also, we excluded the patients with history of long-term sedative drugs (such as oxazepam, diazepam, clonazepam, alprazolam, gabapentin, etc.), history of anti-depressant use (such as sertraline, fluoxetine, fluvoxamine, etc.), any drug abuse, exciter drugs usage (like crystal, ritalin, cocaine, etc.), any sleep disorder detected in tests and non-invasive ventilator extended usage. A total of 51 patients entered our study of which 31% of them were in the DEX group, and 18% were in control group who double-blindly received whether DEX infusion of normal saline 0.9%, and then data were extracted retrospectively according to patients’ file; neither the clinical staffs (consist of anesthesia crew, surgical crew and nursing group), nor data analyzer were aware of the groups until the end of analyze.
We infused DEX, 10min before anesthesia induction in a 0.2–0.6µg/Kg/h. An arterial-line was then fixed in the radial or brachial site. After standard monitoring (consists of a 5-lead EKG, pulse oximetry, and non-invasive blood pressure), the induction of anesthesia was performed using propofol, fentanyl, midazolam, atracurium. Anesthesia was then maintained using an infusion of midazolam and propofol. A routine ABG was sent before induction, 5min after induction, before cross-clamp, during the pump as needed and after disconnecting from the pump and before transferring to ICU. ABG was assessed, and improvement of ventilator tidal volume and respiratory rate was adjusted as needed along with drug administration if needed. An end-tidal CO2 of 35–45 was considered as normal.
DEX infusion was continued post-operatively until weaning from the ventilator. If the patient was not extubated after 24h, it was discontinued due to FDA recommendations on safe administration of DEX. If any drug needed infusion during and after the surgery, the data were documented. The data for hemodynamic (HR, systolic, and diastolic pressure) were documented during and after the surgery, according to the standard sheet. Mean arterial pressure (MAP) was then calculated using the formula and compared before induction and average of post-operative data. Richmond agitation-sedation scale (RASS; Table 1) and critical-care pain observation tool (CPOT; Table 2) was assessed.
Table 1) Richmond Agitation-Sedation Scale

Table 2) The Critical-Care Pain Observation Tool (CPOT)

Table 3) The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU)

Daily routine experiments for all the patients were BUN, creatinine, hematocrit, hemoglobin, platelets count, and WBC. The initial goal of the study was to detect atrial fibrillation (AF) rhythm from EKG interpretation, Acute Kidney Injury (AKI), according to the latest KDIGO guidelines, delirium assessed by the Confusion Assessment Method for Intensive Care Unit (CAM-ICU), mechanical ventilation by minutes and days of ICU stay after surgery (Table 3).
Data were analyzed using SPSS 25 software and expressed as mean (lower bond–upper bond confidence interval 95%) for continuous variables and number (percentage) for categorical variables. Data were then analyzed using one-way ANOVA for continuous data and Chi-square for categorical data.
Findings
We had a total of 51 persons, 33 (64.70%) in the DEX group and 18 (35.29%) in the control group (Table 4).
Table 4) Patients’ characteristics

From the interpretation of the 12 lead EKG of the patients before the surgery, 3 (5.9%) had an AF rhythm of which 2 (66.7%) were in the DEX group and 1 (33.3%) was in the control group (p>0.05). No bradycardia was reported. Only 1 (3%) of the DEX patients had more than 140 beats per minute tachycardia, and no one in the control group had presented with tachycardia (p>0.05). Neither PAC nor PVC rhythm was detected in this research. Also, EKGs indicated no flutter, myocardial infarction, and ischemic heart disease. 28 (63.6%) of the patients with normal cardiac rhythm were in the DEX group, and 16 (36.4%) of them were in our control group (p>0.05).
A total of 27 (52.9%) of the patients had only done CABG surgery, 18 (54.5%) from the DEX group and 9 (50.0%) from the control group (p>0.05; Table 5).
During the surgery, a total of 20 (39.2%) patients required blood products (packed cell, fresh frozen plasma, platelets); 14 (42.4%) of the DEX patients and 6 (33.3%) of the control group (p>0.05). 8 (24.2%) of the DEX group and 3 (16.7%) of the control group needed electroshock due to surgeons’ demand (p>0.05). In the routine ABGs, we detected 2 (6.1%) acidosis in the DEX group and 1 (5.6%) in the control group (p>0.05) from which all three of them needed improvement with sodium bicarbonate (p>0.05) and all of them solved (p>0.05).
Table 5) Perioperative data are described as Mean±Standard deviation or Number (percentage within group)

A total of 25 (49.0%) of the patients needed an infusion of epinephrine, which consists of 14 (42.4%) of the DEX group and 11 (61.1%) of the control group (p>0.05). Also, one patient from each group (3.0% of DEX and 5.6% of the control group) needed norepinephrine (p>0.05). 28 (84.8%) of the DEX group and 17 (94.4%) of the control group received midazolam infusion (p>0.05). Also, 28 (84.8%) of the DEX patients and 16 (88.9%) of the control group sedated with fentanyl drip (p>0.05). Only 2 (6.1%) of the DEX patients needed dopamine, and none of the control group needed (p>0.05). No patient received milrinone. 16 (48.5%) of the DEX group and 11 (40.7%) of the control group required TNG due to hypertension (p>0.05). One of the DEX (3.0%) and one of the control group (5.6%) received furosemide infusion (p>0.05). Mean MAP before surgery for the DEX group was 91.38 (80.84–101.91 CI 95%), and for the control group was 92.44 (85.43–99.45 CI 95%) (p>0.05). After the surgery, during the stay in ICU, the mean MAP was 61.12 (55.43–66.81 CI 95%) in the DEX group and 65.50 (56.23–74.77 CI 95%) in control group (p>0.05).
The mean total mechanical ventilation time was 589.36 (527.66–651.06 CI 95%) minutes in the DEX group and 521.11 (409.50–632.71 CI 95%) minutes in the control group (p>0.05). Total ICU stays in the day have a mean of 2.36 days in the DEX group and 2.77 days in the control group (p>0.05). From our end points, six patients came front with AF ehythm of which only one (16.7%) of them were from the DEX group and the other 5 (83.3%) were from the control group (p=0.009). No patient required dialysis after the surgery. No patient underwent redo surgery, and no one faced delirium. Seven patients seemed to be AKI, which five (71.4%) were in the DEX group, and two (28.6%) were in the control group (p>0.05).
Discussion
This research was designed to investigate the effect of DEX on patients undergoing cardiac surgery. The results did not show any significant difference between the group who received DEX and the control group for AKI; indeed, we had a total of seven AKI patients, which according to KDIGO classification, two of them were stage 2 and no patient from current study became stage 3 AKI. There is a different possibility for this result; first that in our study, we used different drugs, especially midazolam and fentanyl, along with DEX which may affect the renoprotective characteristic of DEX.7 In a study from Ammar et al., creatinine increased significantly higher in the DEX group on day 1. Also, they reported DEX might provide cardiac and renal protection during cardiac surgery though it had no impact on postoperative outcomes [1, 8, 10]. However, some studies suggested that it may have a favorable impact on outcomes in patients with pre-existing cardiac and/or renal dysfunction [11]. In our study, we had excluded patients with any renal complication, and more studies on those patients are recommended. The results contradicted that of Liu et al. whereas they concluded perioperative administration of DEX in adult patients undergoing cardiac surgery might reduce the incidence of post-operative AKI [12]. Future trials are needed to reduce the contradiction in nephrological effects of DEX in cardiac surgery patients.
Another reason that increases the rate of AKI is low blood pressure and/or unstable hemodynamics. The etiology of renal injury is due mainly to the elevation of renin levels as a result of sympathetic overactivity in addition to nephrotoxic, inflammatory, and hemodynamic components. The patients in our study had a mean MAP of 91.38 for the DEX group and 92.44 for the control group before surgery and 61.12 for the DEX group and 65.50 for the control group after surgery. The results were not statistically significant, which was in line with Chang et al. study [13]. Despite this fact, we see a lower MAP in the DEX group, which may explain why we had more AKI in the DEX than in the control group.
Despite diminution in blood pressure, the pain-relieving characteristics of DEX are questionable. In a study about fast-track management in off-pump CABG from Zientara et al., the DEX group needed less use of pain medication in the initial phase at ICU [8]. In the current research, 84.8% of the DEX group and 88.9% of the control group required fentanyl infusion. These results revealed that most of the patients with or without DEX might need another pain-reliever. In our study, we chose to use an infusion of fentanyl instead of any drug stat administration, because the strategy in controlling pain is to prevent it beforehand, instead of healing it.
In contrary to DEX low-potency in relieving pain, it can significantly increase the time of weaning. Mean mechanical ventilation time for the DEX group was 68 min higher compare to the control group; this was contrary to some other studies [8, 14], whereas they used propofol infusion for their control group. Although we used a placebo instead of propofol or any other drugs, for reaching a RASS about -1 to -2, we need to perfuse more of midazolam and fentanyl in the control group. This might explain why, in some cases, DEX group wanted more time for weaning from ventilator. Although DEX has effects on the brain locus coeruleus and the a2-adrenergic receptors of the spinal cord to result in sedation, sympatholytic, analgesia, and anti-nociception, both groups had the same CPOT and RASS in our study. This was in line with some other studies [15]. Besides, DEX did not show any benefit in deep sedation [16], and because of that, we should only consider it as an adjuvant to other drugs. The current research did not show any difference between groups for pain. This was contrary to some studies; among them a meta-analysis from Wang et al. reported that DEX could effectively relieve the pain intensity, extend the pain-free period, and decrease the consumption of opioids during post-operative recovery of adults in general anesthesia [17]; however, their study extracted the data only from one cardiac surgery article. Further, another study in non-cardiac ICU suggests the advantage of DEX in analgesia [18]. Indeed, we recommend a study explicitly designed for assessment of DEX capability to relieve pain in cardiac surgery patients; because the power of sample of our study was according to AKI-occurrence, the results cannot be trusted alone. All the patients experienced a mean ICU stay of about 2 days, and there was no difference between groups from that aspect of view.
The results indicated a meaningful difference between groups for a new-onset AF rhythm after the surgery. This was in line with some other studies [5, 8] and explain the anti-arrhythmic characteristic of DEX [19]. However, a meta-analysis by Zhu et al [20]. revealed that DEX could not reduce the incidence of AF compared to control medicines following cardiac surgery. DEX might have an increased influence on AF occurrence if patients had a history of AF. We had three patients in our study, which had AF rhythm before surgery, but none of them had AF rhythm after surgery.
Most studies suggest that the post-operative administration of DEX may reduce delirium in patients, particularly following cardiac surgery [2, 4, 6, 21]. We used the CAM-ICU tool to screen for delirium based on four features: (1) a fluctuating mental status, (2) inattention, (3) disorganized thinking, and (4) altered level of consciousness. For the determination of delirium, a patient must display features (1) and (2), with either (3) or (4). In the current study, patients had not delirium. This may be a result of having a mean RASS of -2, which indicated a good and deep sleep. Although DEX known to improve the quality of sleep in critically ill patients [3, 22], reduces agitation [23] and reduces pain after cardiothoracic surgery [24, 25], we did not see any difference of RASS and CPOT between groups. This is probably due to the fact that patients from both groups received midazolam and fentanyl as needed. Further studies on DEX anti-arrhythmic effects is suggested.
Conclusion
There might be a meaningful reduction of new-onset AF rhythm in adult patients who use DEX after cardiac surgeries.
Acknowledgements: None declared by the authors.
Ethical Permission: The need for clinical trial registration was waived by the Ethics Committee of the Tehran University of Medical Sciences due to lack of clinical intervention.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Song Y, Kim DH, Kwon TD, Han DW, Baik SH, Jung HH, et al. Effect of intraoperative dexmedetomidine on renal function after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: A randomized, placebo-controlled trial. Int J Hyperthermia. 2019;36(1):1-8. [Link] [DOI:10.1080/02656736.2018.1526416]
2. Cheng H, Li Z, Young N, Boyd D, Atkins Z, Ji F, et al. The effect of dexmedetomidine on outcomes of cardiac surgery in elderly patients. J Cardiothorac Vasc Anesth. 2016;30(6):1502-8. [Link] [DOI:10.1053/j.jvca.2016.02.026]
3. Liu X, Xie G, Zhang K, Song S, Song F, Jin Y, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care. 2017;38:190-6. [Link] [DOI:10.1016/j.jcrc.2016.10.026]
4. Pavone KJ, Cacchione PZ, Polomano RC, Winner L, Compton P. Evaluating the use of dexmedetomidine for the reduction of delirium: An integrative review. Heart Lung. 2018;47(6):591-601. [Link] [DOI:10.1016/j.hrtlng.2018.08.007]
5. Wang G, Niu J, Li Z, Lv H, Cai H. The efficacy and safety of dexmedetomidine in cardiac surgery patients: A systematic review and meta-analysis. PLoS One. 2018;13(9):e0202620. [Link] [DOI:10.1371/journal.pone.0202620]
6. Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127(15):1576-84. [Link] [DOI:10.1161/CIRCULATIONAHA.112.000936]
7. Chen Y, Feng X, Hu X, Sha J, Li B, Zhang H, et al. Dexmedetomidine ameliorates acute stress-induced kidney injury by attenuating oxidative stress and apoptosis through inhibition of the ROS/JNK signaling pathway. Oxid Med Cell Longev. 2018;2018:4035310. [Link] [DOI:10.1155/2018/4035310]
8. Zientara A, Mariotti S, Matter-Ensner S, Seifert B, Graves K, Dzemali O, et al. Fast-track management in off-pump coronary artery bypass grafting: Dexmedetomidine provides rapid extubation and effective pain modulation. Thorac Cardiovasc Surg. 2019;67(6):450-7. [Link] [DOI:10.1055/s-0038-1668602]
9. Erratum: Comparison of the renoprotective effect of dexmedetomidine and dopamine in high-risk renal patients undergoing cardiac surgery: A double-blind randomized study. Ann Card Anaesth. 2018;21(1):108. [Link]
10. Rawat RS, Al Maashani SM. Perioperative renal protection during cardiac surgery: A choice between dopamine and dexmedetomidine. Ann Card Anaesth. 2018;21(1):4-5. [Link]
11. Ammar AS, Mahmoud KM, Kasemy ZA, Helwa MA. Cardiac and renal protective effects of dexmedetomidine in cardiac surgeries: A randomized controlled trial. Saudi J Anaesth. 2016;10(4):395-401. [Link] [DOI:10.4103/1658-354X.177340]
12. Liu Y, Sheng B, Wang S, Lu F, Zhen J, Chen W. Dexmedetomidine prevents acute kidney injury after adult cardiac surgery: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2018;18(1):7. [Link] [DOI:10.1186/s12871-018-0472-1]
13. Chang YF, Chao AN, Shih PY, Hsu YC, Lee CT, Tien YW, et al. Comparison of dexmedetomidine versus propofol on hemodynamics in surgical critically ill patients. J Surg Res. 2018;228:194-200. [Link] [DOI:10.1016/j.jss.2018.03.040]
14. Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med. 2013;6(2):497-502. [Link] [DOI:10.3892/etm.2013.1183]
15. Zhao ZY, Gan JH, Liu JB, Cheng Q. Clinical evaluation of combination of dexmedetomidine and midazolam vs. dexmedetomidine alone for sedation during spinal anesthesia. Saudi J Biol Sci. 2017;24(8):1758-62. [Link] [DOI:10.1016/j.sjbs.2017.11.007]
16. Watt S, Sabouri S, Hegazy R, Gupta P, Heard C. Does dexmedetomidine cause less airway collapse than propofol when used for deep sedation? J Clin Anesth. 2016;35:259-67. [Link] [DOI:10.1016/j.jclinane.2016.07.035]
17. Wang XQ, Liu NF, Chen JL, Xu Z, Wang FM, Ding C. Effect of intravenous dexmedetomidine during general anesthesia on acute postoperative pain in adults: A systematic review and meta-analysis of randomized controlled trials. Clin J Pain. 2018;34(12):1180-91. [Link] [DOI:10.1097/AJP.0000000000000630]
18. Pasero D, Sangalli F, Baiocchi M, Blangetti I, Cattaneo S, Paternoster G, et al. Experienced use of dexmedetomidine in the intensive care unit: A report of a structured consensus. Turk J Anaesthesiol Reanim. 2018;46(3):176-83. [Link] [DOI:10.5152/TJAR.2018.08058]
19. Ling X, Zhou H, Ni Y, Wu C, Zhang C, Zhu Z. Does dexmedetomidine have an antiarrhythmic effect on cardiac patients? A meta-analysis of randomized controlled trials. PLoS One. 2018;13(3):e0193303. [Link] [DOI:10.1371/journal.pone.0193303]
20. Zhu ZP, Zhou HM, Ni YJ, Wu C, Zhang CJ, Ling XY. Can dexmedetomidine reduce atrial fibrillation after cardiac surgery? A systematic review and meta-analysis. Drug Des Dev Ther. 2018;12:521-31. [Link] [DOI:10.2147/DDDT.S153834]
21. Flukiger J, Hollinger A, Speich B, Meier V, Tontsch J, Zehnder T, et al. Dexmedetomidine in prevention and treatment of postoperative and intensive care unit delirium: A systematic review and meta-analysis. Ann Intensive Care. 2018;8(1):92. [Link] [DOI:10.1186/s13613-018-0437-z]
22. Guldenmund P, Vanhaudenhuyse A, Sanders RD, Sleigh J, Bruno MA, Demertzi A, et al. Brain functional connectivity differentiates dexmedetomidine from propofol and natural sleep Br J Anaesth. 2017;119(4):674-84. [Link] [DOI:10.1093/bja/aex257]
23. Lam RPK, Yip WL, Wan CK, Tsui MSH. Dexmedetomidine use in the ED for control of methamphetamine-induced agitation. Am J Emerg Med. 2017;35(4):665.e1-e4. [Link] [DOI:10.1016/j.ajem.2016.11.004]
24. Habibi V, Kiabi FH, Sharifi H. The effect of dexmedetomidine on the acute pain after cardiothoracic surgeries: A systematic review. Braz J Cardiovasc Surg. 2018;33(4):404-17. [Link] [DOI:10.21470/1678-9741-2017-0253]
25. Gallego-Ligorit L, Vives M, Valles-Torres J, Sanjuan-Villarreal TA, Pajares A, Iglesias M. Use of dexmedetomidine in cardiothoracic and vascular anesthesia. J Cardiothor Vasc An. 2018;32(3):1426-38. [Link] [DOI:10.1053/j.jvca.2017.11.044]









