GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 1 (2024)
GMJM 2024, 3(1): 7-11 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/06/15 | Accepted: 2023/12/12 | Published: 2024/01/18
Received: 2023/06/15 | Accepted: 2023/12/12 | Published: 2024/01/18
How to cite this article
Hashemi Feyzabadi S, Davoodi M, Obeidavi Z. Gamma-Oryzanol Alleviated Adverse Effects of DENA-Induced Oxidative Stress in Rat Kidney. GMJM 2024; 3 (1) :7-11
URL: http://gmedicine.de/article-2-216-en.html
URL: http://gmedicine.de/article-2-216-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Semnan University of Medical Sciences, Semnan, Iran
2- Social Determinants of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
3- Lorestan University of Medical Sciences, Khorramabad, Iran
2- Social Determinants of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
3- Lorestan University of Medical Sciences, Khorramabad, Iran
Keywords:
| Abstract (HTML) (1576 Views)
Full-Text: (651 Views)
Introduction
Diethylnitrosamine (DENA) is one carcinogen toxin mainly found in the environment [1]. DENA belongs to the nitrosamine group and causes toxicity in human beings. It is not only found in the environment but also in food products such as dairy, meat, and alcoholic drinks [1]. It induces oxidative stress by releasing free radicals in the environment [2], decreasing the expression of antioxidant enzymes and factors, and decreasing the levels of malondialdehyde [3, 4]. DENA also induces carcinogenesis by inducing injury to macromolecules and the expression of antioxidant genes [5]. It was reported that CCl4 significantly increased the levels of malondialdehyde, decreased the levels of antioxidant factors, and upregulated the gene expressions of the inflammatory cytokines [6]. DENA also induces kidney injuries by increasing oxidative stress. Induction of oxidative stress and enhanced activity of ornithine decarboxylase (ODC) are important promoters for the production of tumors [1]. ODC mainly catalyzes polyamine biosynthetic pathway and is considered a significant target for controlling cancerous cell growth [7]. Oxidative stress induces renal damage, such as acute renal failure, nephropathy glomerular damage, and renal carcinogenesis [8]. Oxidative stress products increase mesangial and endothelial cells, and this work induces changes in the structure and function of the glomerulus [9]. Seemingly, antioxidants can alleviate the adverse effects of DENA on the kidney.
Gamma oryzanol (GO) is a part of the unsaponifiable matter of crude rice bran oil commonly obtained from the rice milling process [10]. The GO is a mixture of ferulic acid esters of phytosterol and triterpene alcohols. Its major compounds are cycloartenol ferulate, 24‐methylene cycloartenol ferulate, sitosterol ferulate, and campesterol [11]. The GO scavenges diphenyl picrylhydrazyl (DPPH), hydroxyl, and superoxide radicals [12].
Due to its antioxidant properties, GO could seem to alleviate the adverse effects of DENA on the kidney. So far, no study has been conducted to evaluate the effects of GO on oxidative stress induced by DENA in rat kidneys. This study was conducted for the first time to evaluate the effects of GO on oxidative stress induced by DENA in rat kidneys.
Materials and Methods
Experimental Design
Sixty male Wistar rats with an initial weight of 180±20g were purchased and kept in the laboratory cages for 1 week. Oxidative stress was induced, as reported by previous studies [13]. Animals were intraperitoneally administrated with DENA (200mg/kg in corn oil) and then subcutaneously administrated with CCl4 (1.0mL/kg) 2 times a week and for 12 weeks. Animals were grouped into 4 groups and 15 rats per group, including Negative control: The rats were subcutaneously administrated with 1mL/kg body weight of corn oil, 2 times/week during the experimental period. Positive control: Rats were administrated with DENA but did not receive any of the treatment. GO-3000 and GO-6000 groups: Rats administrated with DENA and received 3000 and 6000µg/kg GO daily for 12 weeks.
Biochemical estimation in blood and tissues
At the end of the trial, the rats were anesthetized and sacrificed by decapitation. The blood samples were collected from all the animals. Kidney samples were collected for investigation of biochemical analysis. The serum concentrations of urea, creatinine, lipid peroxidation (LPO), reduced glutathione (GSH) level, and activities of the antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) were determined as reported by previous studies [1].
Data analysis
The data were analyzed by SPSS 21 software and reported as the mean ± standard deviation (SD). The ANOVA procedure was used for analysis, and the Tukey test was used for comparison.
Findings
The results for the effects of GO on LPO and GSH are shown in (Figure 1).
The results showed that stress significantly increased LPO and decreased GSH (N-control versus P-control) (p<0.05). The results also showed that the treatment with GO decreased LPO and increased GSH compared to the positive control group (p<0.05). Most of the effect was observed at higher levels.
The results for the serum concentrations of creatinine and urea are illustrated in (Figure 2).
The results showed that the serum concentrations of creatinine and urea were significantly increased in the stressed group (N-control versus P-control) (p<0.05). The results showed the treatment with GO significantly decreased the serum concentrations of creatinine and urea compared to positive control (p<0.05). There was no significant difference between GO-3000 and GO-6000 groups (p>0.05).
The levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase for the effect of stress and GO on antioxidant parameters are shown in (Figure 3).
The results showed that stress significantly increased the levels of SOD, CAT, GPx, and GR (p<0.05) (positive control versus negative control). The results showed that the treatment with GO in both levels significantly increased the levels SOD, CAT, GPx, and GR (p<0.05). There were no significant differences between levels of GO for SOD, GPx, and GR (p>0.05).
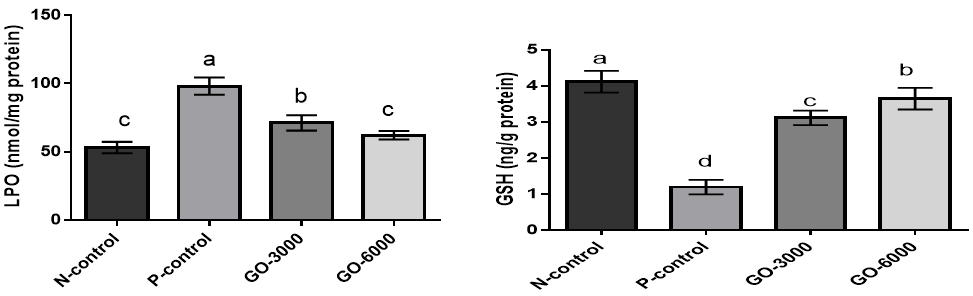
Figure 1. The effects of different GO levels on lipid peroxidation (LPO) and reduced glutathione (GSH) in DENA-induced oxidative stress. Superscripts (a–d) show significant differences among groups. N-control (Negative control), P-control (Positive-control), GO-3000 (gamma-oryzanol 3000) and GO-6000 (gamma-oryzanol 6000). The results showed that stress increased LPO and decreased GSH. The treatment with GO, especially at higher levels, decreased the negative effects of stress on LPO and GSH.
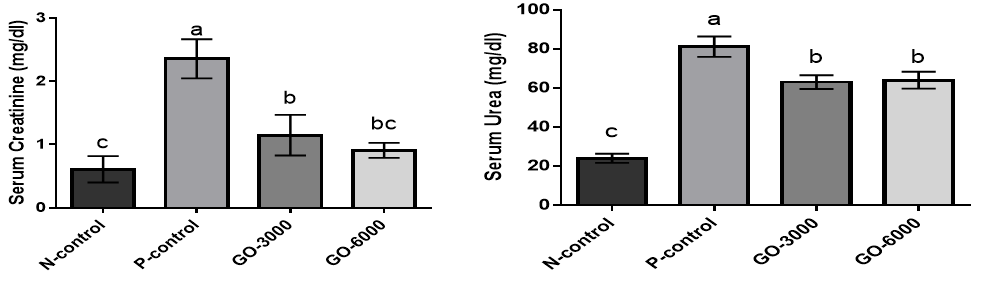
Figure 2. The effects of different GO levels on the serum concentrations of creatinine and urea in DENA-induced oxidative stress. Superscripts (a–c) show significant differences among groups. N-control (Negative control), P-control (Positive-control), GO-3000 (gamma-oryzanol 3000) and GO-6000 (gamma-oryzanol 6000). The results showed that stress increased the serum concentrations of creatinine and urea. The treatment with GO in both levels decreased the negative effects of stress on the serum concentrations of creatinine and urea.
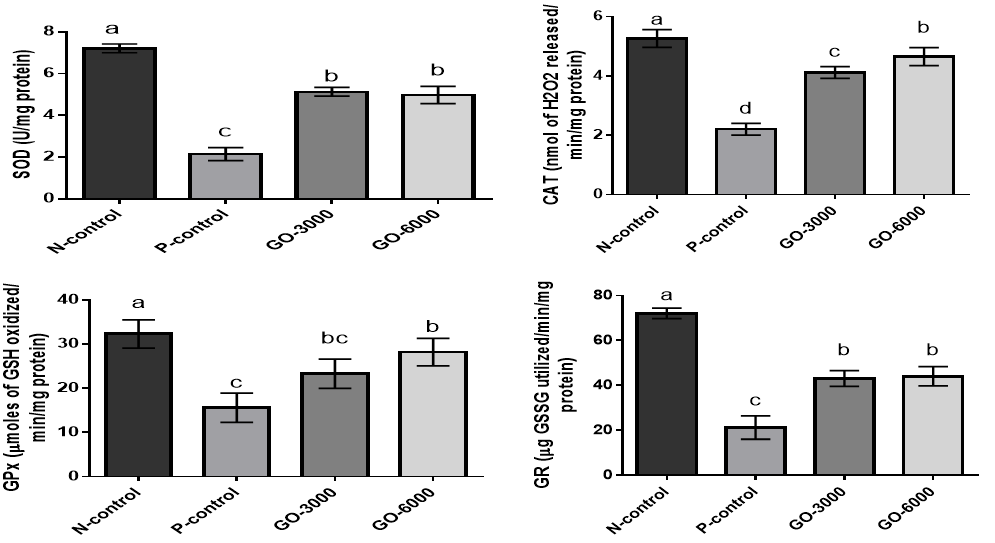
Figure 3. The effects of different levels of GO on superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) on DENA-induced oxidative stress. Superscripts (a–c) show significant differences among groups. N-control (Negative control), P-control (Positive-control), GO-3000 (gamma-oryzanol 3000) and GO-6000 (gamma-oryzanol 6000). The results showed that stress decreased the levels of SOD, CAT, GPx, and GR. The treatment with GO in both levels decreased the negative effects of stress on SOD, CAT, GPx, and GR.
Discussion
DENA is a toxic compound that causes the release of reactive oxygen species (ROS) and induces oxidative stress and cellular damage [14]. Oxidative stress resulting from renal damage causes acute renal failure, disruptive nephropathy, and glomerular damage to chronic renal failure and renal carcinogenesis [8]. The results showed that stress increased LPO. It increases the production of products such as malondialdehyde (MDA) and 4-hydroxynonenal.1 Parallel to our findings, previous studies have shown that DENA increases LPO [15, 16]. It was reported that cytochrome P-450 metabolizes DENA, which results in the production of LPO [17]. Sheweita & Sheikh [18] showed that N-nitrosamines reduced antioxidant levels, and the decrease is associated with the production of free radicals in brain tissues. The treatment with GO significantly decreased LPO due to antioxidant properties. The GO scavenges DPPH, hydroxyl, and superoxide radicals [12] and shows antioxidant properties. Our findings showed that GO increased antioxidant activity by increasing antioxidant factors, including SOD, CAT, GPx, and GR.
The results also showed that DENA increased the levels of creatinine and urea. Similarly, Vargas-Olvera et al. [19] showed that DENA treatment increases oxidative stress, and the reactive oxygen species attack mesangial and endothelial cells, resulting in the structure and function of the glomerulus [20]. These changes cause DENA to bind to the anionic phospholipids in the membrane of the proximal tubular epithelial cells and induce irregularities in the activity and metabolism of the membranes that result in renal failure [21] and increased creatinine and urea. GO prevents damage by antioxidant properties and decreases creatinine and urea levels.
The results also showed that DENA decreased the levels of SOD, CAT, GPx, and GR. A decrease in the antioxidant markers is attributed to decreased expression during renal failure [21] and/or due to N-nitrosamines, which are considered risk factors for brain tumors [18]. GSH is considered an intracellular thiol and has an important role in maintaining free from free radicals and drug detoxification [22]. Since DENA is a toxic electrophilic compound, it might be combined with the nucleophilic site of GSH and thus decrease its macromolecule binding effect [23]. SOD is known as a free radical scavenging enzyme that maintains cells from oxidative stress. It protects the cell against endogenous and exogenous superoxide release [24]. It works with CAT and GPx in a precise manner for scavenging ROS. It activates the alteration of superoxide ion to H2O2, a dangerous free radical that must be converted quickly to water and oxygen by CAT and GPx [24, 25]. In the present study, GO improved antioxidant properties by increasing the levels of SOD, CAT, GPx, and GR, but its mechanism is unknown.
Conclusion
GO could decrease the adverse effects of stress on kidney parameters, and it could be advised to use GO to decrease stress.
Acknowledgments: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Diethylnitrosamine (DENA) is one carcinogen toxin mainly found in the environment [1]. DENA belongs to the nitrosamine group and causes toxicity in human beings. It is not only found in the environment but also in food products such as dairy, meat, and alcoholic drinks [1]. It induces oxidative stress by releasing free radicals in the environment [2], decreasing the expression of antioxidant enzymes and factors, and decreasing the levels of malondialdehyde [3, 4]. DENA also induces carcinogenesis by inducing injury to macromolecules and the expression of antioxidant genes [5]. It was reported that CCl4 significantly increased the levels of malondialdehyde, decreased the levels of antioxidant factors, and upregulated the gene expressions of the inflammatory cytokines [6]. DENA also induces kidney injuries by increasing oxidative stress. Induction of oxidative stress and enhanced activity of ornithine decarboxylase (ODC) are important promoters for the production of tumors [1]. ODC mainly catalyzes polyamine biosynthetic pathway and is considered a significant target for controlling cancerous cell growth [7]. Oxidative stress induces renal damage, such as acute renal failure, nephropathy glomerular damage, and renal carcinogenesis [8]. Oxidative stress products increase mesangial and endothelial cells, and this work induces changes in the structure and function of the glomerulus [9]. Seemingly, antioxidants can alleviate the adverse effects of DENA on the kidney.
Gamma oryzanol (GO) is a part of the unsaponifiable matter of crude rice bran oil commonly obtained from the rice milling process [10]. The GO is a mixture of ferulic acid esters of phytosterol and triterpene alcohols. Its major compounds are cycloartenol ferulate, 24‐methylene cycloartenol ferulate, sitosterol ferulate, and campesterol [11]. The GO scavenges diphenyl picrylhydrazyl (DPPH), hydroxyl, and superoxide radicals [12].
Due to its antioxidant properties, GO could seem to alleviate the adverse effects of DENA on the kidney. So far, no study has been conducted to evaluate the effects of GO on oxidative stress induced by DENA in rat kidneys. This study was conducted for the first time to evaluate the effects of GO on oxidative stress induced by DENA in rat kidneys.
Materials and Methods
Experimental Design
Sixty male Wistar rats with an initial weight of 180±20g were purchased and kept in the laboratory cages for 1 week. Oxidative stress was induced, as reported by previous studies [13]. Animals were intraperitoneally administrated with DENA (200mg/kg in corn oil) and then subcutaneously administrated with CCl4 (1.0mL/kg) 2 times a week and for 12 weeks. Animals were grouped into 4 groups and 15 rats per group, including Negative control: The rats were subcutaneously administrated with 1mL/kg body weight of corn oil, 2 times/week during the experimental period. Positive control: Rats were administrated with DENA but did not receive any of the treatment. GO-3000 and GO-6000 groups: Rats administrated with DENA and received 3000 and 6000µg/kg GO daily for 12 weeks.
Biochemical estimation in blood and tissues
At the end of the trial, the rats were anesthetized and sacrificed by decapitation. The blood samples were collected from all the animals. Kidney samples were collected for investigation of biochemical analysis. The serum concentrations of urea, creatinine, lipid peroxidation (LPO), reduced glutathione (GSH) level, and activities of the antioxidant enzymes (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) were determined as reported by previous studies [1].
Data analysis
The data were analyzed by SPSS 21 software and reported as the mean ± standard deviation (SD). The ANOVA procedure was used for analysis, and the Tukey test was used for comparison.
Findings
The results for the effects of GO on LPO and GSH are shown in (Figure 1).
The results showed that stress significantly increased LPO and decreased GSH (N-control versus P-control) (p<0.05). The results also showed that the treatment with GO decreased LPO and increased GSH compared to the positive control group (p<0.05). Most of the effect was observed at higher levels.
The results for the serum concentrations of creatinine and urea are illustrated in (Figure 2).
The results showed that the serum concentrations of creatinine and urea were significantly increased in the stressed group (N-control versus P-control) (p<0.05). The results showed the treatment with GO significantly decreased the serum concentrations of creatinine and urea compared to positive control (p<0.05). There was no significant difference between GO-3000 and GO-6000 groups (p>0.05).
The levels of superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase for the effect of stress and GO on antioxidant parameters are shown in (Figure 3).
The results showed that stress significantly increased the levels of SOD, CAT, GPx, and GR (p<0.05) (positive control versus negative control). The results showed that the treatment with GO in both levels significantly increased the levels SOD, CAT, GPx, and GR (p<0.05). There were no significant differences between levels of GO for SOD, GPx, and GR (p>0.05).

Figure 1. The effects of different GO levels on lipid peroxidation (LPO) and reduced glutathione (GSH) in DENA-induced oxidative stress. Superscripts (a–d) show significant differences among groups. N-control (Negative control), P-control (Positive-control), GO-3000 (gamma-oryzanol 3000) and GO-6000 (gamma-oryzanol 6000). The results showed that stress increased LPO and decreased GSH. The treatment with GO, especially at higher levels, decreased the negative effects of stress on LPO and GSH.

Figure 2. The effects of different GO levels on the serum concentrations of creatinine and urea in DENA-induced oxidative stress. Superscripts (a–c) show significant differences among groups. N-control (Negative control), P-control (Positive-control), GO-3000 (gamma-oryzanol 3000) and GO-6000 (gamma-oryzanol 6000). The results showed that stress increased the serum concentrations of creatinine and urea. The treatment with GO in both levels decreased the negative effects of stress on the serum concentrations of creatinine and urea.

Figure 3. The effects of different levels of GO on superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) on DENA-induced oxidative stress. Superscripts (a–c) show significant differences among groups. N-control (Negative control), P-control (Positive-control), GO-3000 (gamma-oryzanol 3000) and GO-6000 (gamma-oryzanol 6000). The results showed that stress decreased the levels of SOD, CAT, GPx, and GR. The treatment with GO in both levels decreased the negative effects of stress on SOD, CAT, GPx, and GR.
Discussion
DENA is a toxic compound that causes the release of reactive oxygen species (ROS) and induces oxidative stress and cellular damage [14]. Oxidative stress resulting from renal damage causes acute renal failure, disruptive nephropathy, and glomerular damage to chronic renal failure and renal carcinogenesis [8]. The results showed that stress increased LPO. It increases the production of products such as malondialdehyde (MDA) and 4-hydroxynonenal.1 Parallel to our findings, previous studies have shown that DENA increases LPO [15, 16]. It was reported that cytochrome P-450 metabolizes DENA, which results in the production of LPO [17]. Sheweita & Sheikh [18] showed that N-nitrosamines reduced antioxidant levels, and the decrease is associated with the production of free radicals in brain tissues. The treatment with GO significantly decreased LPO due to antioxidant properties. The GO scavenges DPPH, hydroxyl, and superoxide radicals [12] and shows antioxidant properties. Our findings showed that GO increased antioxidant activity by increasing antioxidant factors, including SOD, CAT, GPx, and GR.
The results also showed that DENA increased the levels of creatinine and urea. Similarly, Vargas-Olvera et al. [19] showed that DENA treatment increases oxidative stress, and the reactive oxygen species attack mesangial and endothelial cells, resulting in the structure and function of the glomerulus [20]. These changes cause DENA to bind to the anionic phospholipids in the membrane of the proximal tubular epithelial cells and induce irregularities in the activity and metabolism of the membranes that result in renal failure [21] and increased creatinine and urea. GO prevents damage by antioxidant properties and decreases creatinine and urea levels.
The results also showed that DENA decreased the levels of SOD, CAT, GPx, and GR. A decrease in the antioxidant markers is attributed to decreased expression during renal failure [21] and/or due to N-nitrosamines, which are considered risk factors for brain tumors [18]. GSH is considered an intracellular thiol and has an important role in maintaining free from free radicals and drug detoxification [22]. Since DENA is a toxic electrophilic compound, it might be combined with the nucleophilic site of GSH and thus decrease its macromolecule binding effect [23]. SOD is known as a free radical scavenging enzyme that maintains cells from oxidative stress. It protects the cell against endogenous and exogenous superoxide release [24]. It works with CAT and GPx in a precise manner for scavenging ROS. It activates the alteration of superoxide ion to H2O2, a dangerous free radical that must be converted quickly to water and oxygen by CAT and GPx [24, 25]. In the present study, GO improved antioxidant properties by increasing the levels of SOD, CAT, GPx, and GR, but its mechanism is unknown.
Conclusion
GO could decrease the adverse effects of stress on kidney parameters, and it could be advised to use GO to decrease stress.
Acknowledgments: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Elguindy NM, Yacout GA, El Azab EF. Amelioration of DENA-induced oxidative stress in rat kidney and brain by the essential oil of Elettaria cardamomum. Beni-Suef Uni J Basic Appl Sci. 2018;7(3):299-305. [Link] [DOI:10.1016/j.bjbas.2018.02.005]
2. Sheweita SA, Mousa N, Al-Masry HM. N-Nitrosodimethylamine changes the expression of glutathione S-transferase in the liver of male mice: the role of antioxidants. J Biochem Mol Toxicol. 2008;22(6):389-95. [Link] [DOI:10.1002/jbt.20255]
3. Bendong C, Mingliang N, Guangshun Y. Effect of paeonol on antioxidant and immune regulatory activity in HCC Rats. Molecule. 2012;17:4672-83. [Link] [DOI:10.3390/molecules17044672]
4. Kadasa NM, Abdallah H, Afifi M, Gowayed S. Hepatoprotective effects of curcumin against diethyl nitrosamine induced hepatotoxicity in Albino rats. Asian Pac J Cancer Prev. 2015;16(1):103-8. [Link] [DOI:10.7314/APJCP.2015.16.1.103]
5. Bansal AK, Bansal M, Soni G, Bhatnagar D. Protective role of vitamin E pretreatment on N-nitrosodiethylamine induced oxidative stress in rat liver. Chem Biol Interact. 2005;156(2-3):101-11. [Link] [DOI:10.1016/j.cbi.2005.08.001]
6. Jia R, Cao L, Du J, Wang J, Liu Y. Effects of carbon tetrachloride on oxidative stress, inflammatory response and hepatocyte apoptosis in common carp (Cyprinus carpio). Aquatic Toxicol. 2014;152:11-9. [Link] [DOI:10.1016/j.aquatox.2014.02.014]
7. Criss WE. A review of polyamines and cancer. Turk J Med Sci. 2003;33(4):195-205. [Link]
8. Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, antioxidant therapies and chronic kidney disease. Nephrol. 2012;17(4):311-21. [Link] [DOI:10.1111/j.1440-1797.2012.01572.x]
9. Begum Q, Noori S, Mahboob T. Antioxidant effect of sodium selenite on thioacetamide-induced renal toxicity. Pak J Biochem Mol Biol. 2011;44(1):21-6. [Link]
10. Lakkakula NR, Lima M, Walker T. Rice bran stabilization and rice bran oil extraction using ohmic heating. Bioresour. Technol. 2004;92(2):157-61. [Link] [DOI:10.1016/j.biortech.2003.08.010]
11. Xu Z, Godber JS. Purification and identification of components of γ‐oryzanol in rice bran oil. J Agr Food Chem. 1999;47:2724‐8. [Link] [DOI:10.1021/jf981175j]
12. Juliano C, Cossu M, Alamanni MC, Piu L. Antioxidant activity of gamma‐oryzanol: mechanism of action and its effect on oxidative stability of pharmaceutical oils. Int. J. Pharm. 2005;299(1-2):146-54. [Link] [DOI:10.1016/j.ijpharm.2005.05.018]
13. Singh BN, Singh BR, Sarma BK, Singh HB. Potential chemoprevention of Nnitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chemico-Biol Interaction. 2009;181(1):20-8. [Link] [DOI:10.1016/j.cbi.2009.05.007]
14. Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33-45. [Link] [DOI:10.1038/ki.2010.337]
15. Mahmoud MA, Ahmed RR, Soliman AH, Salah M. Ruta graveolens and its active constituent rutin protect against diethylnitrosamine-induced nephrotoxicity through modulation of oxidative stress. J Appl Pharm Sci. 2015;5(10):16-21. [Link] [DOI:10.7324/JAPS.2015.501004]
16. Elguindy MN, Yacout AG, El Azab FE, Maghraby KH. Chemoprotective effect of Elettaria cardamomum against chemically induced hepatocellular carcinoma in rats by inhibiting nf-κb, oxidative stress, and activity of ornithine decarboxylase. S Afr J Bot. 2016;105:251-8. [Link] [DOI:10.1016/j.sajb.2016.04.001]
17. Moustafa ME, Mohamed AM, Thabet MN. Gallium nanoparticle-mediated reduction of brain specific serine protease-4 in an experimental metastatic cancer Model. Asian Pac J Cancer Prev. 2017;18(4):895-903. [Link]
18. Sheweita AS, Sheikh YB. Can dietary antioxidants reduce the incidence of brain tumors? Curr Drug Metab. 2011;12(6):587-93. [Link] [DOI:10.2174/138920011795713733]
19. Vargas-Olvera CY, Sánchez-González DJ, Solano JD, Aguilar-Alonso, FA, Montalvo-Muñoz F, Martínez-Martínez CM, et al. Characterization of N-diethylnitrosamine-initiated and ferric nitrilotriacetate- promoted renal cell carcinoma experimental model and effect of a tamarind seed extract against acute nephrotoxicity and carcinogenesis. Mol Cell Biochem. 2012;369(1-2):105-17. [Link] [DOI:10.1007/s11010-012-1373-0]
20. Begum Q, Noori S, Mahboob T. Antioxidant effect of sodium selenite on thioacetamide-induced renal toxicity. Pak J Biochem Mol Biol. 2011;44(1):21-6. [Link]
21. Pracheta P, Sharma V, Singh L, Paliwal R, Sharma S, Yadav S, et al. Chemopreventive effect of hydroethanolic extract of Euphorbia neriifolia leaves against DENA-induced renal carcinogenesis in mice. Asian Pac J Cancer Prev. 2011;12(3):677-83. [Link]
22. Alonso JG, Ros G, Periago MJ. Antiproliferative and cytoprotective activities of a phenolic rich juice in HepG2 cells. Food Res Int. 2006;39(9):982-91. [Link] [DOI:10.1016/j.foodres.2006.07.001]
23. Ketterer B, Meyer DJ. Glutathione-S-transferases: A possible role in the detoxification and repair of DNA and lipid hydroperoxides. Mutat Res. 1989;214(1):33-40. [Link] [DOI:10.1016/0027-5107(89)90195-4]
24. Vasquez-Garzon VR, Arellanes-Robledo J, Garća-Roman R, Aparicio-Rautista DI, Villa-Treviˇno S. Inhibition of reactive oxygen species and preneoplastic lesions by quercetin through an antioxidant defense mechanism. Free Radic Res 2009;43(2):128-37. [Link] [DOI:10.1080/10715760802626535]
25. Sayed-Ahmed MM, Aleisa MA, Al-Rejaie SS, Al-Yahya AA, Al-Shabanah AO, Hafez MM, et al. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev. 2010;3(4):254-61. [Link] [DOI:10.4161/oxim.3.4.12714]









