GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 1 (2024)
GMJM 2024, 3(1): 37-40 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/08/5 | Accepted: 2023/12/29 | Published: 2024/02/10
Received: 2023/08/5 | Accepted: 2023/12/29 | Published: 2024/02/10
How to cite this article
Saboktakin L. Comparison of Serum Zinc Levels in Short and Normal Height Children in Tabriz, Iran. GMJM 2024; 3 (1) :37-40
URL: http://gmedicine.de/article-2-218-en.html
URL: http://gmedicine.de/article-2-218-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
L. Saboktakin *
“Tuberculosis and Lung Disease Research Center” and “Department of Otorhinolaryngology”, Tabriz University of Medical Sciences, Tabriz, Iran
| Abstract (HTML) (1614 Views)
Full-Text: (991 Views)
Introduction
Zinc is an element that is involved in the growth of height. In areas with low zinc intake, short stature is common. Zinc is found in various sources, the most important of which are red meat and poultry [1]. Nuts like walnuts, pistachios, hazelnuts, and almonds are also rich in zinc. Short stature is usually more common in poor areas where consumption of nuts and meat is low due to low income levels. People who do not eat meat either because of an interest in or lack of access to meat or a vegetarian diet should take zinc supplements. Of course, a nutritionist should determine the dose [2, 3].
Zinc is one of the basic micronutrients in the human body, and it is present in the structure of many enzymes that are effective in metabolism and necessary for growth, development, and the central nervous system and immune system [4]. Deficiency in the body reduces growth rate, short stature, and delayed puberty; It also increases the prevalence of infections such as diarrhea and pneumonia. Other symptoms of zinc deficiency include loss of appetite, alopecia and decreased taste and smell, delayed wound healing, chronic diarrhea, and prolonged deficiency of symptoms such as stunted growth and hypogonadism have been reported in men. Zinc deficiency was first reported in 1962 in Iran and Egypt in boys who did not grow up and were not sexually mature [5, 6].
Treatment of mild to moderate deficiencies and supplementation with zinc in children has had beneficial effects in reducing the incidence of diarrhea and pneumonia and even reducing their incidence in areas with a high prevalence [7].
Zinc deficiency has been known in the Middle East for the past three decades and has been present in children in various regions. Studies have been conducted on the prevalence of zinc deficiency in different parts of the world and a few areas in Iran [8].
Due to nutritional differences in different regions, the study of micronutrient deficiencies, including zinc in each region, is very important and useful for that region's medical and health groups. Studies on the high prevalence of zinc deficiency in different parts of Iran: This study was conducted to determine the prevalence of zinc deficiency among six-year-old children with short stature in Tabriz.
Instrument and Methods
This is a cross-sectional descriptive study conducted in 2018 with the participation of 214-year-olds referred to health centers in Tabriz. The sample size was estimated at 214 people based on the number of referrals to health centers during the previous six months, considering the alpha level of 0.05 and the test power of 80%. Detrus sampling was based on the priority of children referring to health centers.
Inclusion criteria: age of six years and satisfaction with the company were rejected. Exclusion criteria also included zinc supplementation during the last six months, children with thyroid disorders, and children with developmental problems.
The consent satisfaction form was completed by the children's parents, and no fees were charged for the children. The weight of children in light clothing and without shoes was measured by a German Soehnle scale with 250g of measuring error, which was adjusted daily by the control weight. The height of barefoot children was measured with a tape measure fixed to the wall and a sack perpendicular to the meter at the top of the head, with the legs glued and the hips, shoulders, and back in contact with the wall. The ratio of weight and height to weight and standard height of the same age is extracted from the NCHS table, and below two standard deviations from the standard population average for weight, weight to height, and height to age are considered underweight, slim, and short height, respectively. Three milliliters of radial venous blood was taken from each child. The samples were diluted evenly using deionized water. Then, the serum zinc level was determined by spectrometry using the Shimatzer apparatus, and those with serum less than 70 micrograms per deciliter were considered zinc deficient.
The collected data were analyzed using SPSS 21 software and displayed as percentages and frequencies or mean and standard deviation. A T-test, one-way analysis of variance, and Chi-squared at the significant level of 0.05 were used to analyze the data.
Findings
This study measured zinc levels in 214 six-year-old children (111 girls and 104 boys). The mean serum zinc level in the study population was 75.56±29.84μg/dl. Zinc deficiency was observed in 118 patients (55.1%). Mean zinc levels in girls and boys were not significantly different (p=0.74).
There was no significant difference in the prevalence and deficiency of zinc and the mean level in girls, boys, short stature, low weight, and thin compared to the population without growth disorders (Table 1).
Table 1. Mean of zinc level in the serum of the studied population, according to demographics
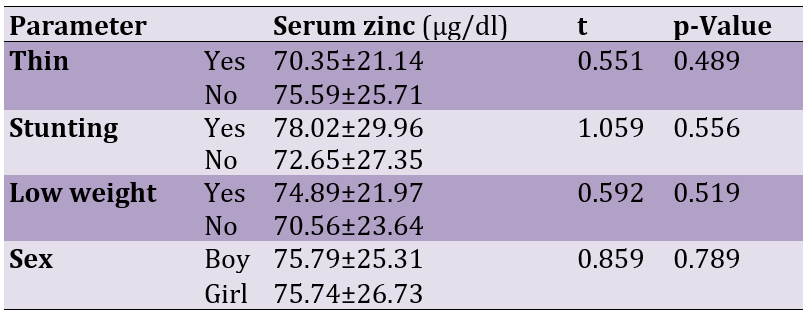
75 participants had zinc deficiency, and the mean zinc level in these subjects was 69.41±25.96μg/dl. The height of these children was significantly lower than that of the group in which the serum zinc level was normal (81.65±31.75μg/dl; p=0.041). On the other hand, the serum zinc level in lean children (59.70±25.41μg/dl) was significantly lower than that of normal-weight children (81.02±34.66μg/dl; p=0.039).
Discussion
Identifying the effective factors in the short stature of children and applying treatment methods such as hormonal corrections and dietary and nutritional corrections has always been of interest to families [9]. Although genetic and hereditary factors can be cited as the main cause of short stature in children, they are not the only possible factor. Growth hormone deficiency, malnutrition in adulthood, chronic diseases such as celiac disease, kidney disease, and diabetes in children are other factors contributing to short stature in children [10, 11].
When parents notice that their children are shorter than their peers and classmates, a wave of anxiety sets in. But for some children, being short is not an abnormality; it is accepted and shows that they are healthy. Genetics is the most important and powerful factor in determining the height of any person. Children can blame their parents, or at least their genes, for their short stature [12, 13].
Before puberty, boys and girls grow at more or less the same speed. On average, babies grow about 10 inches in their first year of birth and an extra 4 inches in their second year. After that, children grow at a declining rate (about 2 inches per year) until puberty. Children reach adulthood at puberty. For girls, puberty usually begins between the ages of 8 and 13, while for boys, it usually starts between 9 and 14. Girls grow about 3 to 3.1 inches per year during their growth spurt during puberty, and boys grow about 4 inches per year during this period. After reaching the peak of their growth, adolescents will still be able to grow their height until they stop growing, approximately four to five years after the growth spurt occurs [14]. Most teens reach adulthood between the ages of 14 and 16, depending on when they reach puberty [15].
In this study, the mean serum zinc level in the population was 75.56+29.84 micrograms per deciliter, which is lower than the standard mean level of approximately 100 micrograms per deciliter [16]. Lack of vitamins and minerals can reduce or stop the growth of children. Children should be provided with a variety of vitamins as they grow. Taking nutritional supplements is one of the best ways to ensure your children get the necessary vitamins and minerals [17]. Supplementation to increase height and bone growth in children is one of the best options to help children grow taller and stronger. Calcium, magnesium, B vitamins, vitamin A, zinc, vitamin D, vitamin C, phosphorus, and … have directly affect height growth. Calcium is essential for bone growth and density. Bones grow during childhood and adolescence, and even after growth stops, bone formation continues but does not cause longitudinal bone growth and height increase. Magnesium stimulates the thyroid gland to secrete the hormone calcitonin, which regulates the amount of calcium in the blood and the activity of bone cells [18, 19]. As magnesium and calcium in the blood increase, bone formation and longitudinal growth increase. Among the B vitamins, vitamins B1 and B2 are more effective in height growth and prevention of short stature than the others [20]. Zinc increases bone growth and height by affecting vitamin D production and, thus, calcium absorption. Therefore, its deficiency will cause acute problems such as short stature [20]. Zinc is a vital mineral that our body uses in countless ways. Zinc is the second most important micro-mineral in the body after iron and is present in every cell. Zinc is needed for the activity of more than 300 enzymes that help with metabolism, digestion, nerve function, and many other processes. In addition, zinc is essential for the growth and function of immune cells. This mineral is also essential for skin health, DNA synthesis, and protein production. Also, the growth and development of the body depend on its role in the growth and division of cells. Zinc is also necessary for our sense of taste and smell. Because one of the most important enzymes for good taste and smell depends on this nutrient, zinc deficiency can reduce our ability to taste or smell. Zinc supplementation can be helpful in children, especially children with anemia. On the other hand, the best source of zinc in the diet is meat and fish. The high consumption of foods high in phytate, such as traditional breads and legumes, which combine almost all of it with phytate, can affect the availability of zinc, and it seems that consumption of this Bread is also high in this area. Therefore, in preventive measures, teaching the correct consumption patterns and improving the eating habits of families can be effective. In addition, broader studies in other age groups and case-control studies are suggested to investigate the effect of supplementation on height and weight of children.
This study's limitations included a lack of knowledge about children's diets and a lack of measurement of serum calcium and other chemical biomarkers in children. These limitations should be addressed in future studies. Also, zinc-containing supplements should be used in children due to the role of zinc in short stature in children.
Conclusion
Zinc deficiency leads to short stature and weight loss in six-year-olds. Therefore, zinc supplements should be considered in children's health planning.
Acknowledgments: None declared by the authors.
Ethical Permissions: The ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1397.870) approved this study, which was coordinated by the Deputy Minister of Health of East Azerbaijan Province.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Zinc is an element that is involved in the growth of height. In areas with low zinc intake, short stature is common. Zinc is found in various sources, the most important of which are red meat and poultry [1]. Nuts like walnuts, pistachios, hazelnuts, and almonds are also rich in zinc. Short stature is usually more common in poor areas where consumption of nuts and meat is low due to low income levels. People who do not eat meat either because of an interest in or lack of access to meat or a vegetarian diet should take zinc supplements. Of course, a nutritionist should determine the dose [2, 3].
Zinc is one of the basic micronutrients in the human body, and it is present in the structure of many enzymes that are effective in metabolism and necessary for growth, development, and the central nervous system and immune system [4]. Deficiency in the body reduces growth rate, short stature, and delayed puberty; It also increases the prevalence of infections such as diarrhea and pneumonia. Other symptoms of zinc deficiency include loss of appetite, alopecia and decreased taste and smell, delayed wound healing, chronic diarrhea, and prolonged deficiency of symptoms such as stunted growth and hypogonadism have been reported in men. Zinc deficiency was first reported in 1962 in Iran and Egypt in boys who did not grow up and were not sexually mature [5, 6].
Treatment of mild to moderate deficiencies and supplementation with zinc in children has had beneficial effects in reducing the incidence of diarrhea and pneumonia and even reducing their incidence in areas with a high prevalence [7].
Zinc deficiency has been known in the Middle East for the past three decades and has been present in children in various regions. Studies have been conducted on the prevalence of zinc deficiency in different parts of the world and a few areas in Iran [8].
Due to nutritional differences in different regions, the study of micronutrient deficiencies, including zinc in each region, is very important and useful for that region's medical and health groups. Studies on the high prevalence of zinc deficiency in different parts of Iran: This study was conducted to determine the prevalence of zinc deficiency among six-year-old children with short stature in Tabriz.
Instrument and Methods
This is a cross-sectional descriptive study conducted in 2018 with the participation of 214-year-olds referred to health centers in Tabriz. The sample size was estimated at 214 people based on the number of referrals to health centers during the previous six months, considering the alpha level of 0.05 and the test power of 80%. Detrus sampling was based on the priority of children referring to health centers.
Inclusion criteria: age of six years and satisfaction with the company were rejected. Exclusion criteria also included zinc supplementation during the last six months, children with thyroid disorders, and children with developmental problems.
The consent satisfaction form was completed by the children's parents, and no fees were charged for the children. The weight of children in light clothing and without shoes was measured by a German Soehnle scale with 250g of measuring error, which was adjusted daily by the control weight. The height of barefoot children was measured with a tape measure fixed to the wall and a sack perpendicular to the meter at the top of the head, with the legs glued and the hips, shoulders, and back in contact with the wall. The ratio of weight and height to weight and standard height of the same age is extracted from the NCHS table, and below two standard deviations from the standard population average for weight, weight to height, and height to age are considered underweight, slim, and short height, respectively. Three milliliters of radial venous blood was taken from each child. The samples were diluted evenly using deionized water. Then, the serum zinc level was determined by spectrometry using the Shimatzer apparatus, and those with serum less than 70 micrograms per deciliter were considered zinc deficient.
The collected data were analyzed using SPSS 21 software and displayed as percentages and frequencies or mean and standard deviation. A T-test, one-way analysis of variance, and Chi-squared at the significant level of 0.05 were used to analyze the data.
Findings
This study measured zinc levels in 214 six-year-old children (111 girls and 104 boys). The mean serum zinc level in the study population was 75.56±29.84μg/dl. Zinc deficiency was observed in 118 patients (55.1%). Mean zinc levels in girls and boys were not significantly different (p=0.74).
There was no significant difference in the prevalence and deficiency of zinc and the mean level in girls, boys, short stature, low weight, and thin compared to the population without growth disorders (Table 1).
Table 1. Mean of zinc level in the serum of the studied population, according to demographics

75 participants had zinc deficiency, and the mean zinc level in these subjects was 69.41±25.96μg/dl. The height of these children was significantly lower than that of the group in which the serum zinc level was normal (81.65±31.75μg/dl; p=0.041). On the other hand, the serum zinc level in lean children (59.70±25.41μg/dl) was significantly lower than that of normal-weight children (81.02±34.66μg/dl; p=0.039).
Discussion
Identifying the effective factors in the short stature of children and applying treatment methods such as hormonal corrections and dietary and nutritional corrections has always been of interest to families [9]. Although genetic and hereditary factors can be cited as the main cause of short stature in children, they are not the only possible factor. Growth hormone deficiency, malnutrition in adulthood, chronic diseases such as celiac disease, kidney disease, and diabetes in children are other factors contributing to short stature in children [10, 11].
When parents notice that their children are shorter than their peers and classmates, a wave of anxiety sets in. But for some children, being short is not an abnormality; it is accepted and shows that they are healthy. Genetics is the most important and powerful factor in determining the height of any person. Children can blame their parents, or at least their genes, for their short stature [12, 13].
Before puberty, boys and girls grow at more or less the same speed. On average, babies grow about 10 inches in their first year of birth and an extra 4 inches in their second year. After that, children grow at a declining rate (about 2 inches per year) until puberty. Children reach adulthood at puberty. For girls, puberty usually begins between the ages of 8 and 13, while for boys, it usually starts between 9 and 14. Girls grow about 3 to 3.1 inches per year during their growth spurt during puberty, and boys grow about 4 inches per year during this period. After reaching the peak of their growth, adolescents will still be able to grow their height until they stop growing, approximately four to five years after the growth spurt occurs [14]. Most teens reach adulthood between the ages of 14 and 16, depending on when they reach puberty [15].
In this study, the mean serum zinc level in the population was 75.56+29.84 micrograms per deciliter, which is lower than the standard mean level of approximately 100 micrograms per deciliter [16]. Lack of vitamins and minerals can reduce or stop the growth of children. Children should be provided with a variety of vitamins as they grow. Taking nutritional supplements is one of the best ways to ensure your children get the necessary vitamins and minerals [17]. Supplementation to increase height and bone growth in children is one of the best options to help children grow taller and stronger. Calcium, magnesium, B vitamins, vitamin A, zinc, vitamin D, vitamin C, phosphorus, and … have directly affect height growth. Calcium is essential for bone growth and density. Bones grow during childhood and adolescence, and even after growth stops, bone formation continues but does not cause longitudinal bone growth and height increase. Magnesium stimulates the thyroid gland to secrete the hormone calcitonin, which regulates the amount of calcium in the blood and the activity of bone cells [18, 19]. As magnesium and calcium in the blood increase, bone formation and longitudinal growth increase. Among the B vitamins, vitamins B1 and B2 are more effective in height growth and prevention of short stature than the others [20]. Zinc increases bone growth and height by affecting vitamin D production and, thus, calcium absorption. Therefore, its deficiency will cause acute problems such as short stature [20]. Zinc is a vital mineral that our body uses in countless ways. Zinc is the second most important micro-mineral in the body after iron and is present in every cell. Zinc is needed for the activity of more than 300 enzymes that help with metabolism, digestion, nerve function, and many other processes. In addition, zinc is essential for the growth and function of immune cells. This mineral is also essential for skin health, DNA synthesis, and protein production. Also, the growth and development of the body depend on its role in the growth and division of cells. Zinc is also necessary for our sense of taste and smell. Because one of the most important enzymes for good taste and smell depends on this nutrient, zinc deficiency can reduce our ability to taste or smell. Zinc supplementation can be helpful in children, especially children with anemia. On the other hand, the best source of zinc in the diet is meat and fish. The high consumption of foods high in phytate, such as traditional breads and legumes, which combine almost all of it with phytate, can affect the availability of zinc, and it seems that consumption of this Bread is also high in this area. Therefore, in preventive measures, teaching the correct consumption patterns and improving the eating habits of families can be effective. In addition, broader studies in other age groups and case-control studies are suggested to investigate the effect of supplementation on height and weight of children.
This study's limitations included a lack of knowledge about children's diets and a lack of measurement of serum calcium and other chemical biomarkers in children. These limitations should be addressed in future studies. Also, zinc-containing supplements should be used in children due to the role of zinc in short stature in children.
Conclusion
Zinc deficiency leads to short stature and weight loss in six-year-olds. Therefore, zinc supplements should be considered in children's health planning.
Acknowledgments: None declared by the authors.
Ethical Permissions: The ethics committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1397.870) approved this study, which was coordinated by the Deputy Minister of Health of East Azerbaijan Province.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Kucukaydin Z, Kurdoglu M, Kurdoglu Z, Demir H, Yoruk IH. Selected maternal, fetal and placental trace element and heavy metal and maternal vitamin levels in preterm deliveries with or without preterm premature rupture of membranes. J Obstet Gynaecol Res. 2018;44(5):880-9. [Link] [DOI:10.1111/jog.13591]
2. Laine JE, Ray P, Bodnar W, Cable PH, Boggess K, Offenbacher S, et al. Placental cadmium levels are associated with increased preeclampsia risk. PLoS One. 2015;10(9):e0139341. [Link] [DOI:10.1371/journal.pone.0139341]
3. Song X, Li B, Li Z, Wang J, Zhang D. High serum copper level is associated with an increased risk of preeclampsia in Asians: A meta-analysis. Nutr Res. 2017;39:14-24. [Link] [DOI:10.1016/j.nutres.2017.01.004]
4. Zhu Q, Zhang L, Chen X, Zhou J, Liu J, Chen J. Association between zinc level and the risk of preeclampsia: a meta-analysis. Arch Gynecol Obstet. 2016;293(2):377-82. [Link] [DOI:10.1007/s00404-015-3883-y]
5. He L, Lang L, Li Y, Liu Q, Yao Y. Comparison of serum zinc, calcium, and magnesium concentrations in women with pregnancy-induced hypertension and healthy pregnant women: A meta-analysis. Hypertens Pregnancy. 2016;35(2):202-9. [Link] [DOI:10.3109/10641955.2015.1137584]
6. Ma Y, Shen X, Zhang D. The relationship between serum zinc level and preeclampsia: A meta-analysis. Nutrients. 2015;7(9):7806-20. [Link] [DOI:10.3390/nu7095366]
7. Ota E, Mori R, Middleton P, Tobe-Gai R, Mahomed K, Miyazaki C, et al. Zinc supplementation for improving pregnancy and infant outcome. Cochrane Database Syst Rev. 2015;2015(2):CD000230. [Link] [DOI:10.1002/14651858.CD000230.pub5]
8. Sabra S, Malmqvist E, Saborit A, Gratacós E, Gomez Roig MD. Heavy metals exposure levels and their correlation with different clinical forms of fetal growth restriction. PLoS ONE. 2017;12(10):e0185645. [Link] [DOI:10.1371/journal.pone.0185645]
9. Gómez T, Bequer L, Mollineda A, González O, Diaz M, Fernández D. Serum zinc levels of cord blood: relation to birth weight and gestational period. J Trace Elem Med Biol. 2015;30:180-3. [Link] [DOI:10.1016/j.jtemb.2014.12.009]
10. Bermúdez L, García-Vicent C, López J, Torró MI, Lurbe E. Assessment of ten trace elements in umbilical cord blood and maternal blood: association with birth weight. J Transl Med. 2015;13;291. [Link] [DOI:10.1186/s12967-015-0654-2]
11. Zhou C, Zhang R, Cai X, Xiao R, Yu H. Trace elements profiles of maternal blood, umbilical cord blood, and placenta in Beijing, China. J Matern Fetal Neonatal Med. 2019;32(11):1755-61. [Link] [DOI:10.1080/14767058.2017.1416602]
12. Terrin G, Berni Canani R, Di Chiara M, Pietravalle A, Aleandri V, Conte F, et al. Zinc in early life: a key element in the fetus and preterm neonate. Nutrients. 2015;7(12):10427-46. [Link] [DOI:10.3390/nu7125542]
13. Boskabadi H, Maamouri G, Mohsen Zadeh H, Shakeri MT, Ghayour-Mobarhan M, Mohammadi S, et al. Comparison of serum zinc level between neonates with jaundice and healthy neonates. Shiraz E-Med J 2015;16(2):34. [Link] [DOI:10.17795/semj27392]
14. Berhe K, Gebrearegay F, Gebremariam H. Prevalence and associated factors of zinc deficiency among pregnant women and children in Ethiopia: a systematic review and meta-analysis. BMC Public Health. 2019;19(1):1663. [Link] [DOI:10.1186/s12889-019-7979-3]
15. Jyotsna S, Amit A, Kumar A. Study of serum zinc in low birth weight neonates and its relation with maternal zinc. J Clin Diagn Res. 2015;9(1):SC01-SC03. [Link] [DOI:10.7860/JCDR/2015/10449.5402]
16. Wilson RL, Grieger JA, Bianco-Miotto T, Roberts C. Association between maternal zinc status, dietary zinc intake and pregnancy complications: A systematic review. Nutrients. 2016;8(10):641. [Link] [DOI:10.3390/nu8100641]
17. Gómez T, Bequer L, Mollineda A, González O, Diaz M, Fernández D. Serum zinc levels of cord blood: relation to birth weight and gestational period. J Trace Elem Med Biol. 2015;30:180-3. [Link] [DOI:10.1016/j.jtemb.2014.12.009]
18. Ramtekkar UP. DSM-5 changes in attention deficit hyperactivity disorder and autism spectrum disorder: implications for comorbid sleep issues. Children. 2017;4(8):62. [Link] [DOI:10.3390/children4080062]
19. Joo H, Choi JH, Burm E, Park H, Hong YC, Kim Y, et al. Gender difference in the effects of lead exposure at different time windows on neurobehavioral development in 5-year-old children. Sci Total Environ. 2018;615:1086-92. [Link] [DOI:10.1016/j.scitotenv.2017.10.007]
20. Elbaz F, Zahra S, Hanafy H. Magnesium, zinc and copper estimation in children with attention deficit hyperactivity disorder (ADHD). Egyptian J Med Human Genet. 2017;18(2):153-63. [Link] [DOI:10.1016/j.ejmhg.2016.04.009]









