GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 2 (2024)
GMJM 2024, 3(2): 69-72 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/11/28 | Accepted: 2023/04/12 | Published: 2024/05/25
Received: 2023/11/28 | Accepted: 2023/04/12 | Published: 2024/05/25
How to cite this article
Liang L, Qili L, Jun W, Yan Y, Hui L, Jie Y. The SPARC Expression and Relationship with Survival in Colorectal Cancer Patients. GMJM 2024; 3 (2) :69-72
URL: http://gmedicine.de/article-2-222-en.html
URL: http://gmedicine.de/article-2-222-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Oncology, Third Affiliated Hospital of Guizhou Medical University, Guizhou province, P.R. China
| Abstract (HTML) (1191 Views)
Full-Text: (715 Views)
Introduction
Colorectal cancer is one of the third most common malignant tumors, and it is the fourth leading cause of cancer-related deaths in the world [1, 2]. In the past few years, CRC incidence and mortality rates have continuously risen. SPARC, also known as bone adhesion protein and basal membrane-40 protein, is a member of the extracellular matrix protein family [3]. The expression of SPARC was first identified in bone and endothelial cells, which played roles in the development and differentiation of chondrocytes and megakaryocytes [4, 5]. SPARC has a wide range of biological effects [6]. SPARC is also expressed in many advanced cancers. Recently, the up-regulated expression of SPARC was associated with gastric cancer, esophageal cancer, and CRC [5, 7, 8], and high levels of SPARC have been shown to be associated with poor prognosis in gastric cancer [8]. SPARC protein directly affects cell adhesion, migration, proliferation, and the formation of blood vessels, plays an important role in the process of the occurrence and development of CRC, and is associated with prognosis in patients [6]. However, the different expression levels in different race groups have not yet been reported. There is no study about the relationship of SPARC with the prognosis of different races. The aim of this study was to analyze the association of SPARC expression in the tissue of Han, Buyi, and Miao CRC patients with clinical-pathological features, DFS, and OS and to explore new possible prognostic and/or predictive biomarkers for Han, Buyi, and Miao CRC patients.
Materials and Methods
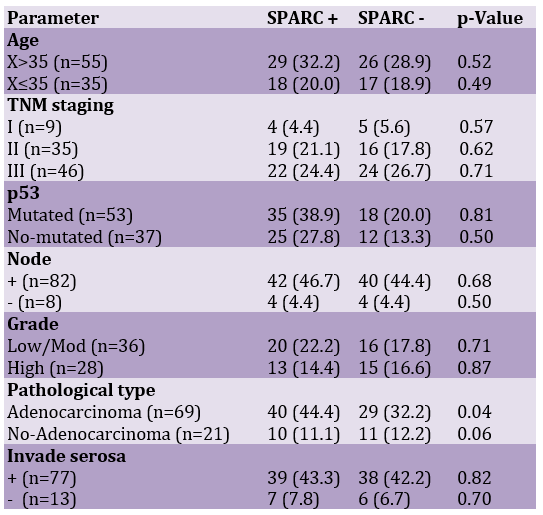
The study examined cases from 101 patients diagnosed between 2006 and 2014 in the Third Hospital affiliated with Guizhou Medical University. 11 cases without evaluable tumor tissue were excluded from the analysis. The final database for analysis included 90 cases with histological confirmation. Clinical data of all the cases were reviewed retrospectively from medical records in our hospital. All patients had a minimum of 5 years of follow-up records. All the patients underwent operational treatment according to clinical practice guidelines of the National Comprehensive Cancer Network (NCCN) of the United States. None of the patients received neo-adjuvant therapy. Statistics and analysis of clinical-pathological parameters, including age at diagnosis, disease stage, tumor size, tumor grade, lymph node status, p53, serosa invasion, and pathological type, were listed in Table 1.
SPARC expression and evaluation of IHC
All tissues were collected surgically under the supervision of an experienced pathologist. IHC measured SPARC expression on FFPE samples. Streptavidin peroxidase (S-P) IHC staining was performed using SPARC antibody of mouse monoclonal (diluted 1/200). The detailed procedures were done as described by Jennbacken [9]. PBS was used to replace the primary antibody in negative controls. According to our data and TMA IHC grading method by Serrero & Ioffe [10] and Pan et al. [11], our scoring was semi-quantitatively categorized as ≤5% of tumor cells staining with/without weakly stained was negative (0), followed by a score of 1 (>5% of tumor cells and with weak/focal positive staining or ≤5% of tumor cells with strongly stained), 2 (>5% of tumor cells and with moderate/focal positive staining), 3 (>5% of tumor cells and with strong/diffuse positive staining).
Table 1. Clinicopathological characteristics of patients
Statistical analysis
The correlation between SPARC, clinical-pathological characteristics, and survival outcomes was compared by Pearson’s χ2 test. Examining the significant difference between the groups with the T-test. Survival analyses, including DFS and OS, were performed with the log-rank test, and all results were displayed in Kaplan–Meier. DFS was defined as the time interval from the date of diagnosis to the time of last disease-free follow-up or at death for those patients who died without a previous recurrence. OS was defined as the time interval from the date of diagnosis to the time of the last follow-up or death [12]. Time to recurrence (local, regional, and distant) was censored at the time of the last disease-free follow-up and at death for those patients who died without a previous recurrence [12]. Statistical significance was defined as p<0.05. The SPSS 17 software package was used for all statistical analyses.
Findings
According to the immunohistochemical results, SPARC is mainly expressed in tumor cell cytoplasm, with a few being nuclear expression. SPARC positive test rate was 52.2% (47/90), including 34.1% (16/47) lower expression and 65.9% (31/47) high expression. There was no difference in the positive expression rate in the Han, Buyi, and Miao races (p=0.87).
To evaluate SPARC prognostic significance, We divided the cases into two groups: one is the high SPARC expression group (SPARC score of 2+ and 3+), and the other one is the low SPARC expression group (SPARC score of 0 and 1+). We analyzed SPARC's different expressions in relation to DFS and OS in CRC patients. There was a marked association with DFS in the Han (p=0.048) and Miao (p=0.047) races (Figure 1). While the same result was not found in the Buyi race (p=0.176). However, no significant difference was found in OS. OS Function curves showed no separation in the Han (p=0.338), Miao (p=0.424), and Buyi (p=0.282) races.
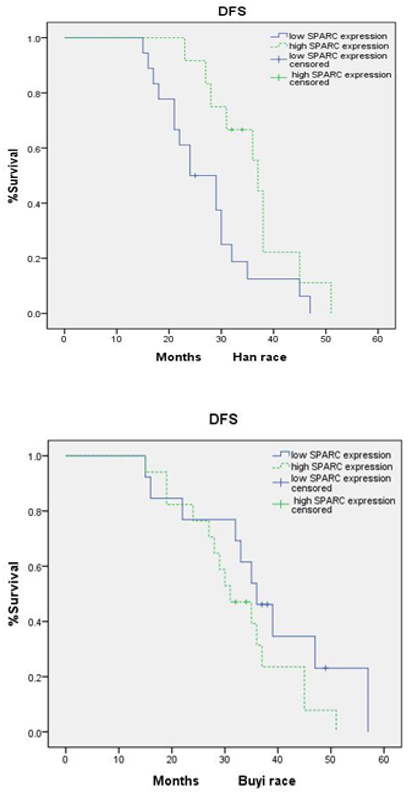
Figure 1. Kaplan-Meier estimates for DFS by high expression group and low expression group in Han, Miao, and Buyi race
Discussion
Colorectal cancer is one of the most common malignant tumors. Many factors are involved in the invasion and metastasis of malignant tumor development. It also studies various adhesion factors, such as hydrolytic enzymes, growth factors, and matrix proteins, which are associated with the invasion and metastasis of tumors. SPARC is a kind of calcium-binding protein. Alexandre & Susan [13] summarized the main function of SPARC. SPARC could destroy cell adhesion, promote cell deformation, inhibit the cell cycle, regulate cell differentiation, inhibit cell response to some growth factors, adjust the extracellular matrix and matrix metalloproteinase, and affect the new angiogenesis. Several studies show that SPARC is associated with the prognosis of CRC. Yang et al. [14] analyzed that SPARC in the lower expression of tumor cells is associated with poor prognosis in 292 cases of primary CRC patients by the 5-year survival rate. Liang et al. [15] found that SPARC expression in CRC tumor stroma is associated with clinical pathological factors, and survival analysis showed that the low SPARC expression in tumor stroma is the cause of the prognosis. In our study, the positive SPARC test rate was 52.2% (47/90), including 34.1% (16/47) lower expression and 65.9% (31/47) high expression. There was no difference in the positive expression rate in the Han, Buyi, and Miao races. SPARC expression was related to the pathological type (Adenocarcinoma) and had no significant correlations with age, nuclear grade, nodal involvement, TNM stage, serosa invasion, and P53. There was a marked association in DFS between the low expression of the SPARC group and the high expression group in the Han and
Miaorace. While the same result was not found in the Buyi race. There was no statistical significance in OS between the low expression of the SPARC group and the high expression group in the Buyi, Miao, and Han races, respectively.
We suggest that SPARC could be a promising prognostic biomarker in CRC patients in the Han and Miao race for cancer prognosis, as well as a possible target for the treatment of these patients.
Conclusion
SPARC is almost the same expression in CRC patients in the Han, buyi, and Miao races by immunostaining, and SPARC may be viewed as a prognostic factor of DFS in the Han and Miao races but not in the Buyi races.
Acknowledgments: None declared by the authors.
Ethical Permission: Approval for this study was obtained from the Third Affiliated Hospital of Guizhou Medical University, Guizhou, China.
Conflicts of Interests: The authors declared no conflict of interest.
Funding/Support: This study was supported by science and technology plan projects in Guizhou and the contract number Qian [2015] 7375.
Colorectal cancer is one of the third most common malignant tumors, and it is the fourth leading cause of cancer-related deaths in the world [1, 2]. In the past few years, CRC incidence and mortality rates have continuously risen. SPARC, also known as bone adhesion protein and basal membrane-40 protein, is a member of the extracellular matrix protein family [3]. The expression of SPARC was first identified in bone and endothelial cells, which played roles in the development and differentiation of chondrocytes and megakaryocytes [4, 5]. SPARC has a wide range of biological effects [6]. SPARC is also expressed in many advanced cancers. Recently, the up-regulated expression of SPARC was associated with gastric cancer, esophageal cancer, and CRC [5, 7, 8], and high levels of SPARC have been shown to be associated with poor prognosis in gastric cancer [8]. SPARC protein directly affects cell adhesion, migration, proliferation, and the formation of blood vessels, plays an important role in the process of the occurrence and development of CRC, and is associated with prognosis in patients [6]. However, the different expression levels in different race groups have not yet been reported. There is no study about the relationship of SPARC with the prognosis of different races. The aim of this study was to analyze the association of SPARC expression in the tissue of Han, Buyi, and Miao CRC patients with clinical-pathological features, DFS, and OS and to explore new possible prognostic and/or predictive biomarkers for Han, Buyi, and Miao CRC patients.
Materials and Methods
The study examined cases from 101 patients diagnosed between 2006 and 2014 in the Third Hospital affiliated with Guizhou Medical University. 11 cases without evaluable tumor tissue were excluded from the analysis. The final database for analysis included 90 cases with histological confirmation. Clinical data of all the cases were reviewed retrospectively from medical records in our hospital. All patients had a minimum of 5 years of follow-up records. All the patients underwent operational treatment according to clinical practice guidelines of the National Comprehensive Cancer Network (NCCN) of the United States. None of the patients received neo-adjuvant therapy. Statistics and analysis of clinical-pathological parameters, including age at diagnosis, disease stage, tumor size, tumor grade, lymph node status, p53, serosa invasion, and pathological type, were listed in Table 1.
SPARC expression and evaluation of IHC
All tissues were collected surgically under the supervision of an experienced pathologist. IHC measured SPARC expression on FFPE samples. Streptavidin peroxidase (S-P) IHC staining was performed using SPARC antibody of mouse monoclonal (diluted 1/200). The detailed procedures were done as described by Jennbacken [9]. PBS was used to replace the primary antibody in negative controls. According to our data and TMA IHC grading method by Serrero & Ioffe [10] and Pan et al. [11], our scoring was semi-quantitatively categorized as ≤5% of tumor cells staining with/without weakly stained was negative (0), followed by a score of 1 (>5% of tumor cells and with weak/focal positive staining or ≤5% of tumor cells with strongly stained), 2 (>5% of tumor cells and with moderate/focal positive staining), 3 (>5% of tumor cells and with strong/diffuse positive staining).
Table 1. Clinicopathological characteristics of patients

Statistical analysis
The correlation between SPARC, clinical-pathological characteristics, and survival outcomes was compared by Pearson’s χ2 test. Examining the significant difference between the groups with the T-test. Survival analyses, including DFS and OS, were performed with the log-rank test, and all results were displayed in Kaplan–Meier. DFS was defined as the time interval from the date of diagnosis to the time of last disease-free follow-up or at death for those patients who died without a previous recurrence. OS was defined as the time interval from the date of diagnosis to the time of the last follow-up or death [12]. Time to recurrence (local, regional, and distant) was censored at the time of the last disease-free follow-up and at death for those patients who died without a previous recurrence [12]. Statistical significance was defined as p<0.05. The SPSS 17 software package was used for all statistical analyses.
Findings
According to the immunohistochemical results, SPARC is mainly expressed in tumor cell cytoplasm, with a few being nuclear expression. SPARC positive test rate was 52.2% (47/90), including 34.1% (16/47) lower expression and 65.9% (31/47) high expression. There was no difference in the positive expression rate in the Han, Buyi, and Miao races (p=0.87).
To evaluate SPARC prognostic significance, We divided the cases into two groups: one is the high SPARC expression group (SPARC score of 2+ and 3+), and the other one is the low SPARC expression group (SPARC score of 0 and 1+). We analyzed SPARC's different expressions in relation to DFS and OS in CRC patients. There was a marked association with DFS in the Han (p=0.048) and Miao (p=0.047) races (Figure 1). While the same result was not found in the Buyi race (p=0.176). However, no significant difference was found in OS. OS Function curves showed no separation in the Han (p=0.338), Miao (p=0.424), and Buyi (p=0.282) races.

Figure 1. Kaplan-Meier estimates for DFS by high expression group and low expression group in Han, Miao, and Buyi race
Discussion
Colorectal cancer is one of the most common malignant tumors. Many factors are involved in the invasion and metastasis of malignant tumor development. It also studies various adhesion factors, such as hydrolytic enzymes, growth factors, and matrix proteins, which are associated with the invasion and metastasis of tumors. SPARC is a kind of calcium-binding protein. Alexandre & Susan [13] summarized the main function of SPARC. SPARC could destroy cell adhesion, promote cell deformation, inhibit the cell cycle, regulate cell differentiation, inhibit cell response to some growth factors, adjust the extracellular matrix and matrix metalloproteinase, and affect the new angiogenesis. Several studies show that SPARC is associated with the prognosis of CRC. Yang et al. [14] analyzed that SPARC in the lower expression of tumor cells is associated with poor prognosis in 292 cases of primary CRC patients by the 5-year survival rate. Liang et al. [15] found that SPARC expression in CRC tumor stroma is associated with clinical pathological factors, and survival analysis showed that the low SPARC expression in tumor stroma is the cause of the prognosis. In our study, the positive SPARC test rate was 52.2% (47/90), including 34.1% (16/47) lower expression and 65.9% (31/47) high expression. There was no difference in the positive expression rate in the Han, Buyi, and Miao races. SPARC expression was related to the pathological type (Adenocarcinoma) and had no significant correlations with age, nuclear grade, nodal involvement, TNM stage, serosa invasion, and P53. There was a marked association in DFS between the low expression of the SPARC group and the high expression group in the Han and
Miaorace. While the same result was not found in the Buyi race. There was no statistical significance in OS between the low expression of the SPARC group and the high expression group in the Buyi, Miao, and Han races, respectively.
We suggest that SPARC could be a promising prognostic biomarker in CRC patients in the Han and Miao race for cancer prognosis, as well as a possible target for the treatment of these patients.
Conclusion
SPARC is almost the same expression in CRC patients in the Han, buyi, and Miao races by immunostaining, and SPARC may be viewed as a prognostic factor of DFS in the Han and Miao races but not in the Buyi races.
Acknowledgments: None declared by the authors.
Ethical Permission: Approval for this study was obtained from the Third Affiliated Hospital of Guizhou Medical University, Guizhou, China.
Conflicts of Interests: The authors declared no conflict of interest.
Funding/Support: This study was supported by science and technology plan projects in Guizhou and the contract number Qian [2015] 7375.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [Link] [DOI:10.3322/caac.20107]
2. Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490-502. [Link] [DOI:10.1016/S0140-6736(13)61649-9]
3. Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29(2):295-307. [Link] [DOI:10.1007/s10555-010-9221-8]
4. Tai IT, Tang MJ. SPARC in cancer biology: Its role in cancer progression and potential for therapy. Drug Resist Updat. 2008;11(6): 231-46. [Link] [DOI:10.1016/j.drup.2008.08.005]
5. Takemasa I, Higuchi H, Yamamoto H, Sekimoto M, Tomita N, Nakamori S, et al. Construction of preferential cDNA microarray specialized for human colorectal carcinoma: molecular sketch of colorectal cancer. Biochem Biophys Res Commun. 2001;285(5):1244-9. [Link] [DOI:10.1006/bbrc.2001.5277]
6. Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21(1):55-65. [Link] [DOI:10.1016/j.semcdb.2009.11.018]
7. Brabender J, Lord RV, Metzger R, Park J, Salonga D, Danenberg KD, et al. Differential SPARC mRNA expression in Barrett's oesophagus. Br J Cancer. 2003;89(8):1508-12. [Link] [DOI:10.1038/sj.bjc.6601324]
8. Wang CS, Lin KH, Chen SL, Chan YF, Hsueh S. Over expression of SPARC gene in human gastric carcinoma and its clinic-pathologic significance. Br J Cancer. 2004;91(11):1924-30. [Link] [DOI:10.1038/sj.bjc.6602213]
9. Jennbacken K, Vallbo C, Wang W, Damber JE. Expression of Vascular Endothelial Growth Factor C (VEGF-C) and VEGF Receptor-3 in human prostate cancer are associated with regional lymph node metastasis. Prostate. 2005;65(2):110-6. [Link] [DOI:10.1002/pros.20276]
10. Serrero G, Ioffe O. Expression of the novel autocrine growth factor PC-Cell Derived Growth Factor in human breast cancer tissue. Hum Pathol. 2003;34(11):1148-54. [Link] [DOI:10.1016/S0046-8177(03)00425-8]
11. Pan AP, Huang GY, Chen J. Relationship between hepatitis B virus covalently closed circular DNA and HBx protein expression in hepatocellular carcinoma and its significance. World Chin J Digestol. 2009;17:712-5. [Link] [DOI:10.11569/wcjd.v17.i7.712]
12. Serrero G, Hawkins DM, Yue B, Ioffe O, Bejarano P, Phillips JT, et al. Progranulin (GP88) tumor tissue expression is associated with increased risk of recurrence in breast cancer patients diagnosed with estrogen receptor positive invasive ductal carcinoma. Breast Cancer Res. 2012;14(1):R26. [Link] [DOI:10.1186/bcr3111]
13. Alexandre C, Susan LC. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21(1):55-65. [Link] [DOI:10.1016/j.semcdb.2009.11.018]
14. Yang E, Kang HJ, Koh KH, Rhee H, Kim NK, Kim H. Frequent inactivation of SPARC by promoter hypermethylation in colon cancers. Int J Cancer. 2007;121(3):567-75. [Link] [DOI:10.1002/ijc.22706]
15. Liang JF, Wang HK, Xiao H, Li N, Cheng CX, Zhao YZ, et al. Relationship and prognostic significance of SPARC and VEGF protein expression in colon cancer. J Exp Clin Cancer Res. 2010;29:71. [Link] [DOI:10.1186/1756-9966-29-71]









