GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 4 (2024)
GMJM 2024, 3(4): 133-137 |
Back to browse issues page
History
Received: 2024/05/3 | Accepted: 2024/10/15 | Published: 2024/12/7
Received: 2024/05/3 | Accepted: 2024/10/15 | Published: 2024/12/7
How to cite this article
Bakhtiari A, Aboudzadeh S, Vaziri S, Mirzaei Roozbahani M. Efficiency of Probiotics in Immobilized Rats through Involvement of the Antioxidant and Anti-Inflammatory Systems. GMJM 2024; 3 (4) :133-137
URL: http://gmedicine.de/article-2-235-en.html
URL: http://gmedicine.de/article-2-235-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Internal Medicine, Lorestan University of Medical Sciences, Khorramabad, Iran
2- Imam Ali Health Center, Abadan University of Medical Sciences, Abadan, Iran
3- Student Research Committee, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
4- Department of General Surgery, Lorestan University of medical Sciences, Khorramabad, Iran
2- Imam Ali Health Center, Abadan University of Medical Sciences, Abadan, Iran
3- Student Research Committee, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
4- Department of General Surgery, Lorestan University of medical Sciences, Khorramabad, Iran
Keywords:
| Abstract (HTML) (1517 Views)
Full-Text: (387 Views)
Introduction
Stress is known as a factor that causes negative effects on general physiology and leads to psychology [1] and heart and causes diabetes and loss of weight [2]. It also causes faulted working memory, anxiety, and depression [3]. Studies have accepted the role of stress in suppression of immunity [4] and intestinal microflora [5]. It also causes the production of free radicals and oxidative damage to macromolecules [6]. Stress also increases the production of pro-inflammatory cytokines such as transcription factor NF-κB-mediated pathways and other inflammatory mediators [7].
In addition, stress is related to increased oxidative stress that stimulates phosphorylation of mitogen-activated protein kinases in animal models [8, 9]. Immobilization is a type of physical stress that limits mobilization and increases aggression in the animal model [2]. It is also used to study stress-induced changes [10].
Experimental studies that have shown probiotics' possible effects on the host's psychological status are rare. The gastrointestinal tract is an active metabolic organ containing various microbial species. It has been accepted that decreased healthy microflora provides an opportunity for pathogenic bacteria and increases inflammation [11]. It is shown that specific probiotic strains reduce the production of pro-inflammatory cytokines by decreasing the integrity of the tight junctional complexes between epithelial cells [12, 13].
Probiotic lactobacilli and bifidobacteria's profitable role in inflammatory conditions has been reported [14]. A study has also shown that probiotics significantly decrease the basal levels of some oxidative stress markers and increase the power of antioxidant enzymes, showing the antioxidant properties of probiotics [15]. Immobilization is the type of stress that influences inflammation and oxidative stress. On the other hand, probiotics can positively affect oxidative stress and inflammation. So far, no study has been conducted to evaluate the effects of probiotics on immobility stress. This study was thus conducted to evaluate the effects of probiotics in immobilized rats through the involvement of antioxidant and anti-inflammatory systems.
Materials and Methods
Materials
Commercial kits of interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), nitric oxide (NO), corticosterone, transforming growth factor-beta (TGF-β), malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx) were purchased from Sigma Aldrich Company. Commercial strains of Lactobacillus plantarum and Bifidobacterium B94 were selected to test.
Animals
72 Albino Wistar rats (6 weeks-age, 170±10g) were adapted for one week before trial and kept based on the animal welfare laws. All the animals were maintained at an optimal temperature (25±1°C), and humidity (55±5%) and illumination period (12 h light and 12 h dark) were kept in the experimental period. Animals received ad libitum water and feed. Animals were exposed to stress to induce immobilization stress, as reported by others [16]. Animals were exposed to immobilization for 2h/d and two weeks by a restraining chamber [17]. On days 7 and 14 of the trial, animals were anesthetized, blood samples were collected, and sera were collected.
Groups
Animals were divided into three groups (n=24), each divided into four sub-groups. Groups were included: 1) Normal control group without stress (Control), 2) Immobilized rats without additive (Immobilized), 3) Immobilized rats given 104 CFU probiotics.
Inflammatory cytokines and antioxidant status
Inflammatory and pro-inflammatory cytokines, including IL-10, TNF-α, TGF-β, NO, and corticosterone, were measured on days 7 and 14. Commercial kits evaluated the MDA, SOD, and GPx concentrations in serum according to the manufacturers’ instructions on days 7 and 14.
Statistical Analysis
The obtained data were analyzed using Graph Pad Prism 7.0 (GraphPad Software, Inc., 7825 Fay Avenue, Suite 230, La Jolla, CA, USA) and reported as mean±standard deviation. Groups were compared by the Tukey test.
Findings
Corticostrone, MDA and NO concentrations
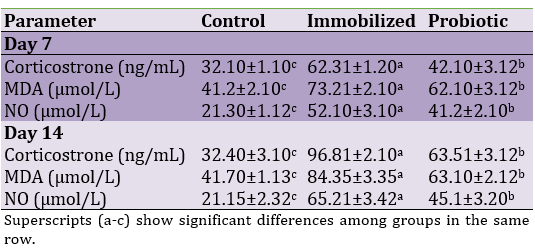
Immobilization could significantly increase corticosterone, MDA, and NO serum concentrations on days 7 and 14, as immobilized rats were compared with control rats (p<0.05). However, the administration of probiotics could significantly decrease corticosterone, MDA, and NO (p<0.05; Table 1).
Table 1. Effects of experimental treatments on serum concentrations of corticosterone, MDA, and NO in immobilized rats
Inflammatory factors
Our findings for inflammatory responses are presented in Figure 1. Results showed that exposure to immobilization increased levels of TNF-α and TGF-β and decreased levels of IL-10 in days 7 and 14 (p<0.05). Treatment with probiotics could significantly decrease TNF-α and TGF-β and increase levels of IL-10 in days 7 and 14 (p<0.05).
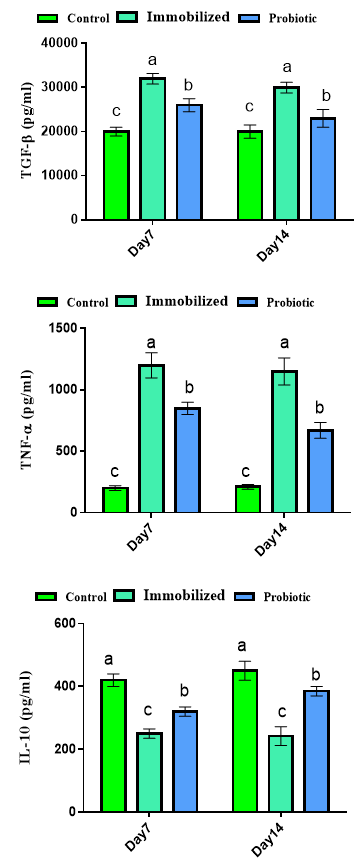
Figure 1. Effects of experimental treatments on inflammatory responses in immobilized rats. Superscripts (a-c) show significant differences among groups on the same day.
Antioxidant enzymes
Our findings for antioxidant status are shown in Figure 2. Results showed that the serum concentrations of SOD and GPx were decreased in immobilized rats, but treatment with probiotics could significantly improve antioxidant status in days 7 and 14 (p<0.05).
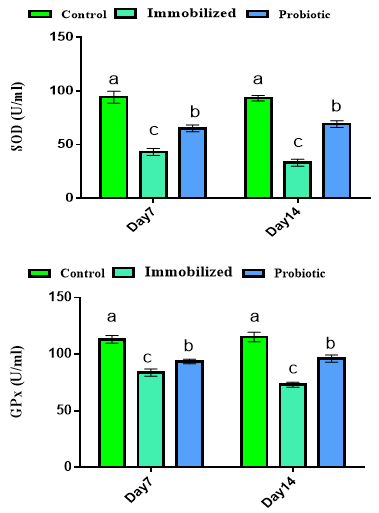
Figure 2. Effects of experimental treatments on antioxidant status in immobilized rats. Superscripts (a-c) show significant differences among groups on the same day.
Discussion
Immobility stress increased levels of MDA, NO, and corticosterone. It has been accepted that stress increases the production of reactive oxygen species by biological system ability [18] and increases MDA production as a marker for lipid peroxidation. MDA shows increased oxidative damage and reduced antioxidants. It has been reported that there is a relationship between stress, increased corticosterone, and intestinal injuries [19]. Stress changes the neuroendocrine system by activating the hypothalamic-pituitary-adrenal axis and increasing the production of corticosterone [20]. Stress increases corticostrone production and changes structural proteins [21]. It has been reported that MDA and NO are inflammatory biomarkers [22]. On the other hand, oxidative stress plays a major role in the pathogenesis of immobilized stress [23]. Improved MDA and NO were observed in probiotic groups. Improved MDA and NO could be attributed to antioxidant and anti-inflammatory responses, which will be discussed later. It has been reported that probiotic strains of L. rhamnosus [24], Enterococcus faecium [15], and L. acidophilus [25] improve antioxidant capacity, which could be attributed to microbial metabolic activity during fermentation [26]. Increased inflammatory responses were observed in rats exposed to stress. It has been reported that stress increases corticostrone, and increased corticostrone decreases mRNA expression of anti-inflammatory cytokines, i.e., IL-10 [27], as observed in the current study. IL-10 is known as one of the most important anti-inflammatory cytokines, which prevent the production of pro-inflammatory cytokines such as TNF-α [28]. IL-10 has been reported to have anti-inflammatory properties that are formed by both T-cells and monocytes/macrophages. The TNF-α has been known as an inflammatory cytokine that initiates inflammation. It means that immobilization stress increases inflammation, but probiotics decrease inflammation. Stress provides an opportunity for pathogens that increase inflammation. Previous studies have reported that L. fermentum could significantly decrease jejunal inflammation of the upper small intestine after 5-FU administration in rats [29]. Improved anti-inflammatory properties could be attributed to reduced pro-inflammatory cytokines, increased immature leukocyte production, and interferon production [30, 31]. Probiotics also decrease pathogen-induced inflammation that is caused to improve the intestinal ecosystem. It could be stated that probiotics improve inflammation conditions by the mentioned mechanisms. Stress also decreased levels of antioxidant enzymes. Stress stimulates and increases the metabolic rate, which causes high free radicals and oxidative damage [6]. Stress decreases antioxidant enzymes, but probiotics improve them. Antioxidant properties of probiotics have previously been reported [15], but the mechanism is unknown.
Conclusion
Similar to other stresses, immobilization increased inflammation and oxidation. Probiotics could improve inflammatory responses and have antioxidant properties. It could be recommended to use probiotics as a daily supplement in patients with stress-related conditions, such as depression.
Acknowledgments: None declared by the authors.
Ethical Permission: There were no ethical considerations in this research.
Conflicts of Interests: The authors declared no conflict of interest.
Funding/Support: This research did not receive a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Stress is known as a factor that causes negative effects on general physiology and leads to psychology [1] and heart and causes diabetes and loss of weight [2]. It also causes faulted working memory, anxiety, and depression [3]. Studies have accepted the role of stress in suppression of immunity [4] and intestinal microflora [5]. It also causes the production of free radicals and oxidative damage to macromolecules [6]. Stress also increases the production of pro-inflammatory cytokines such as transcription factor NF-κB-mediated pathways and other inflammatory mediators [7].
In addition, stress is related to increased oxidative stress that stimulates phosphorylation of mitogen-activated protein kinases in animal models [8, 9]. Immobilization is a type of physical stress that limits mobilization and increases aggression in the animal model [2]. It is also used to study stress-induced changes [10].
Experimental studies that have shown probiotics' possible effects on the host's psychological status are rare. The gastrointestinal tract is an active metabolic organ containing various microbial species. It has been accepted that decreased healthy microflora provides an opportunity for pathogenic bacteria and increases inflammation [11]. It is shown that specific probiotic strains reduce the production of pro-inflammatory cytokines by decreasing the integrity of the tight junctional complexes between epithelial cells [12, 13].
Probiotic lactobacilli and bifidobacteria's profitable role in inflammatory conditions has been reported [14]. A study has also shown that probiotics significantly decrease the basal levels of some oxidative stress markers and increase the power of antioxidant enzymes, showing the antioxidant properties of probiotics [15]. Immobilization is the type of stress that influences inflammation and oxidative stress. On the other hand, probiotics can positively affect oxidative stress and inflammation. So far, no study has been conducted to evaluate the effects of probiotics on immobility stress. This study was thus conducted to evaluate the effects of probiotics in immobilized rats through the involvement of antioxidant and anti-inflammatory systems.
Materials and Methods
Materials
Commercial kits of interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α), nitric oxide (NO), corticosterone, transforming growth factor-beta (TGF-β), malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GPx) were purchased from Sigma Aldrich Company. Commercial strains of Lactobacillus plantarum and Bifidobacterium B94 were selected to test.
Animals
72 Albino Wistar rats (6 weeks-age, 170±10g) were adapted for one week before trial and kept based on the animal welfare laws. All the animals were maintained at an optimal temperature (25±1°C), and humidity (55±5%) and illumination period (12 h light and 12 h dark) were kept in the experimental period. Animals received ad libitum water and feed. Animals were exposed to stress to induce immobilization stress, as reported by others [16]. Animals were exposed to immobilization for 2h/d and two weeks by a restraining chamber [17]. On days 7 and 14 of the trial, animals were anesthetized, blood samples were collected, and sera were collected.
Groups
Animals were divided into three groups (n=24), each divided into four sub-groups. Groups were included: 1) Normal control group without stress (Control), 2) Immobilized rats without additive (Immobilized), 3) Immobilized rats given 104 CFU probiotics.
Inflammatory cytokines and antioxidant status
Inflammatory and pro-inflammatory cytokines, including IL-10, TNF-α, TGF-β, NO, and corticosterone, were measured on days 7 and 14. Commercial kits evaluated the MDA, SOD, and GPx concentrations in serum according to the manufacturers’ instructions on days 7 and 14.
Statistical Analysis
The obtained data were analyzed using Graph Pad Prism 7.0 (GraphPad Software, Inc., 7825 Fay Avenue, Suite 230, La Jolla, CA, USA) and reported as mean±standard deviation. Groups were compared by the Tukey test.
Findings
Corticostrone, MDA and NO concentrations
Immobilization could significantly increase corticosterone, MDA, and NO serum concentrations on days 7 and 14, as immobilized rats were compared with control rats (p<0.05). However, the administration of probiotics could significantly decrease corticosterone, MDA, and NO (p<0.05; Table 1).
Table 1. Effects of experimental treatments on serum concentrations of corticosterone, MDA, and NO in immobilized rats

Inflammatory factors
Our findings for inflammatory responses are presented in Figure 1. Results showed that exposure to immobilization increased levels of TNF-α and TGF-β and decreased levels of IL-10 in days 7 and 14 (p<0.05). Treatment with probiotics could significantly decrease TNF-α and TGF-β and increase levels of IL-10 in days 7 and 14 (p<0.05).

Figure 1. Effects of experimental treatments on inflammatory responses in immobilized rats. Superscripts (a-c) show significant differences among groups on the same day.
Antioxidant enzymes
Our findings for antioxidant status are shown in Figure 2. Results showed that the serum concentrations of SOD and GPx were decreased in immobilized rats, but treatment with probiotics could significantly improve antioxidant status in days 7 and 14 (p<0.05).

Figure 2. Effects of experimental treatments on antioxidant status in immobilized rats. Superscripts (a-c) show significant differences among groups on the same day.
Discussion
Immobility stress increased levels of MDA, NO, and corticosterone. It has been accepted that stress increases the production of reactive oxygen species by biological system ability [18] and increases MDA production as a marker for lipid peroxidation. MDA shows increased oxidative damage and reduced antioxidants. It has been reported that there is a relationship between stress, increased corticosterone, and intestinal injuries [19]. Stress changes the neuroendocrine system by activating the hypothalamic-pituitary-adrenal axis and increasing the production of corticosterone [20]. Stress increases corticostrone production and changes structural proteins [21]. It has been reported that MDA and NO are inflammatory biomarkers [22]. On the other hand, oxidative stress plays a major role in the pathogenesis of immobilized stress [23]. Improved MDA and NO were observed in probiotic groups. Improved MDA and NO could be attributed to antioxidant and anti-inflammatory responses, which will be discussed later. It has been reported that probiotic strains of L. rhamnosus [24], Enterococcus faecium [15], and L. acidophilus [25] improve antioxidant capacity, which could be attributed to microbial metabolic activity during fermentation [26]. Increased inflammatory responses were observed in rats exposed to stress. It has been reported that stress increases corticostrone, and increased corticostrone decreases mRNA expression of anti-inflammatory cytokines, i.e., IL-10 [27], as observed in the current study. IL-10 is known as one of the most important anti-inflammatory cytokines, which prevent the production of pro-inflammatory cytokines such as TNF-α [28]. IL-10 has been reported to have anti-inflammatory properties that are formed by both T-cells and monocytes/macrophages. The TNF-α has been known as an inflammatory cytokine that initiates inflammation. It means that immobilization stress increases inflammation, but probiotics decrease inflammation. Stress provides an opportunity for pathogens that increase inflammation. Previous studies have reported that L. fermentum could significantly decrease jejunal inflammation of the upper small intestine after 5-FU administration in rats [29]. Improved anti-inflammatory properties could be attributed to reduced pro-inflammatory cytokines, increased immature leukocyte production, and interferon production [30, 31]. Probiotics also decrease pathogen-induced inflammation that is caused to improve the intestinal ecosystem. It could be stated that probiotics improve inflammation conditions by the mentioned mechanisms. Stress also decreased levels of antioxidant enzymes. Stress stimulates and increases the metabolic rate, which causes high free radicals and oxidative damage [6]. Stress decreases antioxidant enzymes, but probiotics improve them. Antioxidant properties of probiotics have previously been reported [15], but the mechanism is unknown.
Conclusion
Similar to other stresses, immobilization increased inflammation and oxidation. Probiotics could improve inflammatory responses and have antioxidant properties. It could be recommended to use probiotics as a daily supplement in patients with stress-related conditions, such as depression.
Acknowledgments: None declared by the authors.
Ethical Permission: There were no ethical considerations in this research.
Conflicts of Interests: The authors declared no conflict of interest.
Funding/Support: This research did not receive a specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. Sheikh N, Ahmad A, Siripurapu KB, Kuchibhotla VK, Singh S, Palit G. Effect of Bacopa monniera on stress-induced changes in plasma corticosterone and brain monoamines in rats. J Ethnopharmacol. 2007;111:671-6. [Link] [DOI:10.1016/j.jep.2007.01.025]
2. Ramadan KS, Alshamrani SA. Effects of Salvadora persica extract on the hematological and biochemical alterations against immobilization-induced rats. Scientifica. 2015;2015:253195. [Link] [DOI:10.1155/2015/253195]
3. Patki, G, Solanki, N, Atrooz, F, Allam, F, Salim, S. Depression, anxiety-like behavior, and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res. 2013;1539:73-86. [Link] [DOI:10.1016/j.brainres.2013.09.033]
4. Viswanathan K, Dhabhar FS. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proc Natl Acad Sci USA. 2005;102:5808-13. [Link] [DOI:10.1073/pnas.0501650102]
5. Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397-407. [Link] [DOI:10.1016/j.bbi.2010.10.023]
6. Halliwell B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet. 1994;344:721-4. [Link] [DOI:10.1016/S0140-6736(94)92211-X]
7. Madrigal JL, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Bosca L, Leza JC. Inducible nitric oxide synthase expression in the brain cortex after acute restraint stress is regulated by nuclear factor κB-mediated mechanisms. J Neurochem. 2001;76:532-8. [Link] [DOI:10.1046/j.1471-4159.2001.00108.x]
8. Sasaguri K, Kikuchi M, Hori N, Yuyama N, Onozuka M, Sato S. Suppression of stress immobilization-induced phosphorylation of ERK 1/2 by biting in the rat hypothalamic paraventricular nucleus. Neurosci Lett. 2005;383:160-4. [Link] [DOI:10.1016/j.neulet.2005.04.011]
9. Hebert MA, Serova LI, Sabban EL. Single and repeated immobilization stress differentially triggers induction and phosphorylation of several transcription factors and mitogen-activated protein kinases in the rat locus coeruleus. J Neurochem. 2005;95:484-98. [Link] [DOI:10.1111/j.1471-4159.2005.03386.x]
10. Ahmad A, Rasheed N, Chand K, Maurya R, Banu N, Palit G. Restraint stress-induced central monoaminergic and oxidative changes in rats and their prevention by novel Ocimum sanctum compounds. Indian J Med Res. 2012;135:548-54. [Link]
11. Honad K, Littman DR. The microbiome in infectious disease and inflammation. Ann Rev Immunol.2012;30:758-95. [Link] [DOI:10.1146/annurev-immunol-020711-074937]
12. Heyman M, Terpend K, Menard S. Effects of specific lactic acid bacteria on the intestinal permeability to macromolecules and the inflammatory condition. Acta Paediatr Suppl. 2005;94(449):34-6. [Link] [DOI:10.1111/j.1651-2227.2005.tb02153.x]
13. Donato KA, Gareau M, Wang YJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumor necrosis factor-a-induced barrier dysfunction and pro-inflammatory signaling. Microbiol. 2010;156:3288-97. [Link] [DOI:10.1099/mic.0.040139-0]
14. Okada Y, Tsuzuki Y, Hokari R, Komoto S, Kurihara C, Kawaguchi A, et al. Anti-inflammatory effects of the genus Bifidobacterium on macrophages by modification of phosphor-IkB and SOCS gene expression. Int J Exp Pathol. 2009;90:131-40. [Link] [DOI:10.1111/j.1365-2613.2008.00632.x]
15. Divyashri G, Krishna G, Muralidhara B, Prapulla SG. Probiotic attributes, antioxidant, anti-inflammatory and neuromodulatory effects of Enterococcus faecium CFR 3003: In vitro and in vivo evidence. J Med Microbiol. 2015;64:1527-40. [Link] [DOI:10.1099/jmm.0.000184]
16. Yang HJ, Kim KY, Kang P, Lee HS, Seol GH. Effects of Salvia sclarea on chronic immobilization stress induced endothelial dysfunction in rats. BMC Complement Altern Med. 2014;14:396. [Link] [DOI:10.1186/1472-6882-14-396]
17. Rubisz-Brzezinska J, Jonderko G, Zebracka T, Dyczek-Parys E. Immunoglobulin E levels in selected dermatoses. PrzDermatol. 1977;64:17-22. [Link]
18. Samarghandian S, Azimi-Nezhad M, Samini F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exper Anim. 2015;64:65-71. [Link] [DOI:10.1538/expanim.14-0027]
19. Olfati A, Mojtahedin A, Sadeghi T, Akbari M, Martínez-Pastor F. Comparison of growth performance and immune responses of broiler chicks reared under heat stress, cold stress and thermoneutral conditions. Spanish J Agric Res. 2018;16:1-7. [Link] [DOI:10.5424/sjar/2018162-12753]
20. Quinteiro-Filho WM, Gomes AV, Pinheiro ML, Ribeiro A, Ferraz-de-Paula V, Astolfi-Ferreira CS, et al. Heat stress impairs performance and induces intestinal inflammation in broiler chickens infected with Salmonella enteritidis. Avi Pathol.2012;41:421-7. [Link] [DOI:10.1080/03079457.2012.709315]
21. Lin H, Decuypere E, Buyse J. Oxidative stress induced by corticosterone administration in broiler chickens (Gallus gallus domesticus) 1. Chronic exposure. Comp Biochem Physiol. 2004;139:737-44. [Link] [DOI:10.1016/j.cbpc.2004.09.013]
22. Fiore R, Miller R, Coffman SM. Mycobacterium mucogenicum infection following a cosmeticprocedure with poly-L-lactic acid. J Drugs Dermatol. 2013;12:353-7. [Link]
23. Zafir A, Banu N. Induction of oxidative stress by restraint stress and corticosterone treatments in rats. Ind J Biochem Biophys. 2009;46:53-8. [Link]
24. Marazza JA, Nazareno MA, de Giori GS, Garro MS. Enhance the antioxidant capacity ofsoymilk by fermentation with Lactobacillus rhamnosus. J Funct Food.2012;4:594-601. [Link] [DOI:10.1016/j.jff.2012.03.005]
25. Mousavi ZE, Mousavi SM, Razavi SH, Hadinejad M, Emam-Djomeh Z, Mirzapour M. Effect of fermentation of pomegranate juice by lactobacillus plantarum and lactobacillus acidophilus on the antioxidant activity and metabolism of sugars, organic acids, and phenolic compounds. Food Biotechnol.2013;27:1-13. [Link] [DOI:10.1080/08905436.2012.724037]
26. Tamang JP, Shin DH, Jung SJ, Chae SW. Functional Properties of Microorganisms in Fermented Foods. Front Microbiol. 2016;7:578. [Link] [DOI:10.3389/fmicb.2016.00578]
27. Quinteiro-Filho WM, Calefi AS, Cruz DSG, Aloia TPA, Zager A, Astolfi-Ferreira CS, et al. Heat stress decreases expression of the cytokines, avian β-defensins 4 and 6, and Toll-like receptor 2 in broiler chickens infected with Salmonella enteritidis. Vet Immunol Immunopathol. 2017;186:19-28. [Link] [DOI:10.1016/j.vetimm.2017.02.006]
28. de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med.1991;174(5):1209-20. [Link] [DOI:10.1084/jem.174.5.1209]
29. Smith CL, Geier MS, Yazbeck R, Torres DM, Butler RN, Howarth GS. Lactobacillus fermentumBR11 and fructooligosaccharide partially reduce jejunal inflammation in a model of intestinal mucositis in rats. Nutr Cancer. 2008;60(6):757-67. [Link] [DOI:10.1080/01635580802192841]
30. Park SY, Kim YH, Kim EK, Ryu EY, Lee SJ. Hemeoxygenase-1 signals are involved in preferential inhibiting pro-inflammatory cytokine release by surfactin in cells activated with Porphyromonas gingivalislipopolysaccharide. Chem Biol Interact. 2010;188(3):437-45. [Link] [DOI:10.1016/j.cbi.2010.09.007]
31. Brunt J, Austin B. The development of probiotics for controlling multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis. 2007;30(10):573-9. [Link] [DOI:10.1111/j.1365-2761.2007.00836.x]









