GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 4 (2024)
GMJM 2024, 3(4): 127-131 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/04/12 | Accepted: 2024/09/18 | Published: 2024/11/8
Received: 2024/04/12 | Accepted: 2024/09/18 | Published: 2024/11/8
How to cite this article
Kheirollahi A, Hatami S, Olfat A. Relationship between Novel Markers and Sperm Quality in Obese Rats. GMJM 2024; 3 (4) :127-131
URL: http://gmedicine.de/article-2-237-en.html
URL: http://gmedicine.de/article-2-237-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Lorestan University of Medical Sciences, Khorramabad, Iran
2- Laboratory of Ecological Animal Science and Environmental Veterinary Medicine, College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu, China
2- Laboratory of Ecological Animal Science and Environmental Veterinary Medicine, College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu, China
Keywords:
| Abstract (HTML) (1416 Views)
Full-Text: (380 Views)
Introduction
Obesity has been known as a common metabolic disorder that occurs due to an imbalance between energy consumption and expenditure. It has been known as one of the major problems for healthiness [1]. It has been known to affect lipid metabolic processes such as lipogenesis and lipolysis [2]. Obesity affects fertility and the male reproductive system by its negative effect on erectile dysfunction and semen variables [3]. It has been known to cause higher infertility in obese men [4]. Obesity has been known to have stimulator effects on reproduction in male individuals that could be attributed to increased reactive oxygen species (ROS) [5].
Infertile men have been shown high levels of ROS in semen that could be attributed to increased levels of pro-inflammatory cytokines and leucocyte invasion in their semen [6]. On the other hand, inflammation is known as a natural host response against microbial attack or tissue damage that restores tissue vasculature and functions [7, 8]. It has been shown that inflammation increased spermatogenic arrest and prevented processes of sperm maturation [4]. Inflammation specifically influences spermatocytes and spermatids but not spermatogonia [9]. Stress oxidative influences lipid parameters, and obese individuals experience hyperlipidemia. Lipids have been known to play a significant role in the functional activity of sperm cells [10, 11]. Sperm viability, maturity, capacitation, and fertilization are influenced by lipid parameters [12]. Phospholipids and cholesterol are known as important variables of human plasma membranes, and they are needed for membrane permeability, fluidity, and capacitation [13]. Tumor necrosis factor-α (TNF-α) is one pro-inflammatory cytokine in the borderline of the inflammation process. Interleukin-1β (IL-1β), another pro-inflammatory cytokine, stimulates neutrophils into the region of infection [14]. Nuclear factor-kappa B (NF-κB) increases the production of TNF-α and IL-1β [14]. C-reactive protein (CRP) is one inflammatory marker associated with increased systemic inflammatory response syndrome [15]. Sialic acid has been reported as an inflammatory cytokine, and its levels are increased during inflammation [16]. Haptoglobin (Hp) is another factor that scavenges hemoglobin to be released into circulation by hemolysis or normal red blood cell (RBC) turnover [17].
So far, no study has evaluated the relationship between the mentioned markers and fertility in obese animals. This study was thus conducted to assess the relationship between novel markers and sperm quality in obese rats.
Materials and Methods
Animals
Thirty-six male Wistar rats (6 weeks of age, 210±10g) were acclimatized for one week before trial and maintained based on animal welfare laws. All the animals were kept at an optimal temperature (25±1°C), and humidity (55±5%) and illumination period (12h light and 12h dark) were kept in the experimental period. Standard pellets were prepared from Javaneh Khorasan Company-Iran, and cholesterol, cholic acid, and palm oil were purchased for high-fat diets. Animals received ad libitum water and feed. Animals were grouped into two groups (n=18), each divided into three sub-groups. The normal group received a normal rat pellet diet for 12 weeks of intervention, while the other group received a high-fat diet. Food consumption and body weight were evaluated on days 1, 42, and 84. At the end of the trial, animals were sacrificed after an overnight fast, and their blood samples were stored for evaluation of sera.
The serum concentration of pro-inflammatory cytokines
The serum concentrations of IL-1β (Abcam, No.Ab197742), IL-6 (Abcam, No. Ab100713), IL-3 (Abcam, No. Ab113345), TNF-α (Abcam, No. Ab6671), sialic acid (Abcam, No. Ab83375), CRP (Cayman Chemical, No. 10011236), haptoglobin (Abcam, No. Ab108856) and fibrinogen (AssayMax) were measured as recommended by producer Companies. The serum concentrations of triglycerides and cholesterol were assessed by commercial kits (Pars Azmoon; Iran).
Sperm parameters
At the end of the trial, following euthanasia, the right vas deferens were gathered. Sperm were collected by one syringe and needle using internal rinsing with 1.0ml of modified HTF medium (Human Tubal Fluid, Irvine Scientific) at 34°C. A Makler counting chamber heated up to 34°C was included with a small aliquot of sperm solution. Assessment of sperm motility was conducted by one person in all the trials and calculated by visual estimation (100 spermatozoa per animal, in duplicate) under a phase-contrast microscope (Leica DMLS) in 200X magnification. Spermatozoa were grouped as immotile, motile, lacking in progression, and motile, containing progressive movement. Sperm were also removed from the left vas deferens using internal rinsing with 1.0ml of saline formula by a syringe and needle. To evaluate the sperm morphology, smears were made histological slides left to dry for 90 minutes and observed in a phase-contrast microscope (400×magnification) [18], and 200 spermatozoa were assessed per rat. Morphological abnormalities were classified into two general categories, as reported by others [19].
Statistical Analysis
As the data was normal, all the analyses were conducted using the T-test, and correlation was conducted using Pearson correlation in SPSS 20 software. Figures were illustrated using Graph Pad Prism.
Findings
Body weight
Results in Figure 1 showed that experimental treatments did not influence body weight on day 1 (p>0.05). Still, body weight was significantly higher in rats fed high-fat diets compared to the control group on days 42 and 84 (p<0.05).
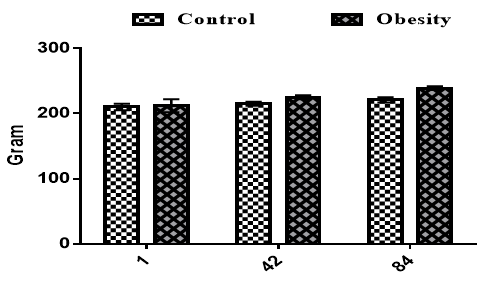
Figure 1. Effects of obesity on body weight on the different days in the rats
Lipid profile
The serum concentrations of cholesterol and triglycerides were significantly higher in obese rats compared to the control group (p<0.05; Figure 2).
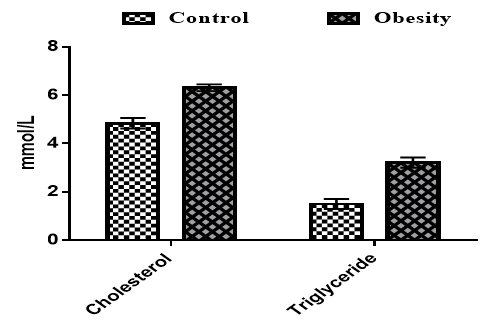
Figure 2. Effects of obesity on the serum concentrations of cholesterol and triglycerides in the rats
Sperm quality
Our findings for sperm quality are shown in Figure 3. Results showed that progressive motility was significantly higher in the control group compared to obese rats (p<0.05). The non-progressive motility and immotility were significantly higher in obese rats compared to control groups (p<0.05).
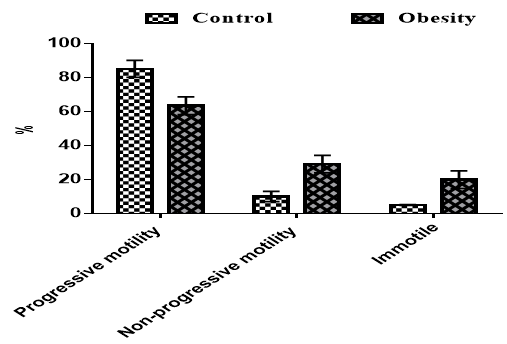
Figure 3. Effects of obesity on sperm quality in rats
Inflammatory factors
The effects of obesity on inflammatory factors are shown in Table 1.
Table 1. Effects of experimental treatments on inflammatory markers (all significant at 0.05)
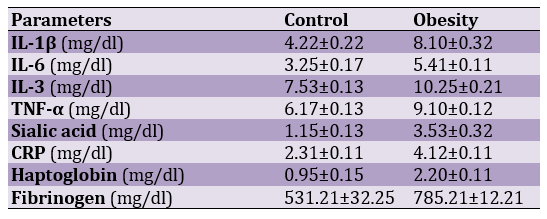
IL-1β, IL-6, IL-3, TNF-α, Sialic acid, CRP, Haptoglobin, and Fibrinogen were significantly higher in obese rats than in control rats (p<0.05). There was a negative correlation between progressive motility and a positive correlation between non-progressive motility and immotile with inflammatory factors (p<0.05).
Discussion
The diets used for obesity were efficient in promoting obesity, as highlighted by enhanced body weight. Increased body weight could be attributed to a hyperlipidemic diet. It has been accepted that consumption of high-fat diets raises oxidation. Increased oxidation enhances lipid deposition, i.e., lipid profile, which increases body weight [20, 21]. Our findings showed an increased lipid profile in obese rats, which confirms obesity. Increased oxidation during obesity could be attributed to oxidation, which increases lipolysis and lipid profile. Unfortunately, we did not evaluate oxidation parameters, but our findings for lipid parameters confirm obesity.
With regards to sperm quality, it was negatively influenced by experimental treatments. Sperm motility has been known as one of the most common variables used to assess sperm quality [22-24]. Sperm motility is obtained during sperm transition by the epididymal duct [25-29]. Previous studies have shown decreased motile sperm without changes in testosterone levels [30]. Morphological changes in obese animals show a high probability that obesity adversely influences spermatogenesis. It has been accepted that spermatogenesis is influenced in males with extreme obesity [31]. Our findings confirmed the adverse effects of obesity on sperm quality.
Increased inflammatory markers were observed in obese rats. Adipose tissue produces TNF-α and interleukins that are essential to energy regulation.
The ROS formed during inflammation is well thought-out as a factor, and amplified mitochondrial ROS production may have a significant role in the pathogenesis of obesity [32]. Metabolic inflammation is a major factor of obesity [33], and inflammatory signaling could considerably influence lipid metabolism in the liver [34]. TNF-α initiates the inflammation process, and then IL-1β, another pro-inflammatory cytokine, calls neutrophils into the region of infection [14]. CRP increases inflammatory responses [15]. Sialic acid and Hp increase during inflammation [16, 17]. We observed a relationship between inflammatory markers and sperm quality. Inflammation has been reported as a natural host response against microbial attack or tissue damage that restores tissue vasculature and functions [7, 8]. Inflammation increased spermatogenic arrest and inhibited processes of sperm maturation [4]. Inflammation specifically influences spermatocytes and spermatids but not spermatogonia [9]. So far, studies have not investigated the relationship between these factors and sperm quality. We could not find any study showing the relation between sperm quality and pro-inflammatory factors.
Conclusion
There is a positive relationship between obesity and lipid profile. Results also showed increased serum concentrations of IL-1β, IL-6, IL-3, TNF-α, sialic acid, CRP, haptoglobin, and fibrinogen in obese rats. Increased factors are correlated with decreased sperm quality.
Acknowledgments: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
Obesity has been known as a common metabolic disorder that occurs due to an imbalance between energy consumption and expenditure. It has been known as one of the major problems for healthiness [1]. It has been known to affect lipid metabolic processes such as lipogenesis and lipolysis [2]. Obesity affects fertility and the male reproductive system by its negative effect on erectile dysfunction and semen variables [3]. It has been known to cause higher infertility in obese men [4]. Obesity has been known to have stimulator effects on reproduction in male individuals that could be attributed to increased reactive oxygen species (ROS) [5].
Infertile men have been shown high levels of ROS in semen that could be attributed to increased levels of pro-inflammatory cytokines and leucocyte invasion in their semen [6]. On the other hand, inflammation is known as a natural host response against microbial attack or tissue damage that restores tissue vasculature and functions [7, 8]. It has been shown that inflammation increased spermatogenic arrest and prevented processes of sperm maturation [4]. Inflammation specifically influences spermatocytes and spermatids but not spermatogonia [9]. Stress oxidative influences lipid parameters, and obese individuals experience hyperlipidemia. Lipids have been known to play a significant role in the functional activity of sperm cells [10, 11]. Sperm viability, maturity, capacitation, and fertilization are influenced by lipid parameters [12]. Phospholipids and cholesterol are known as important variables of human plasma membranes, and they are needed for membrane permeability, fluidity, and capacitation [13]. Tumor necrosis factor-α (TNF-α) is one pro-inflammatory cytokine in the borderline of the inflammation process. Interleukin-1β (IL-1β), another pro-inflammatory cytokine, stimulates neutrophils into the region of infection [14]. Nuclear factor-kappa B (NF-κB) increases the production of TNF-α and IL-1β [14]. C-reactive protein (CRP) is one inflammatory marker associated with increased systemic inflammatory response syndrome [15]. Sialic acid has been reported as an inflammatory cytokine, and its levels are increased during inflammation [16]. Haptoglobin (Hp) is another factor that scavenges hemoglobin to be released into circulation by hemolysis or normal red blood cell (RBC) turnover [17].
So far, no study has evaluated the relationship between the mentioned markers and fertility in obese animals. This study was thus conducted to assess the relationship between novel markers and sperm quality in obese rats.
Materials and Methods
Animals
Thirty-six male Wistar rats (6 weeks of age, 210±10g) were acclimatized for one week before trial and maintained based on animal welfare laws. All the animals were kept at an optimal temperature (25±1°C), and humidity (55±5%) and illumination period (12h light and 12h dark) were kept in the experimental period. Standard pellets were prepared from Javaneh Khorasan Company-Iran, and cholesterol, cholic acid, and palm oil were purchased for high-fat diets. Animals received ad libitum water and feed. Animals were grouped into two groups (n=18), each divided into three sub-groups. The normal group received a normal rat pellet diet for 12 weeks of intervention, while the other group received a high-fat diet. Food consumption and body weight were evaluated on days 1, 42, and 84. At the end of the trial, animals were sacrificed after an overnight fast, and their blood samples were stored for evaluation of sera.
The serum concentration of pro-inflammatory cytokines
The serum concentrations of IL-1β (Abcam, No.Ab197742), IL-6 (Abcam, No. Ab100713), IL-3 (Abcam, No. Ab113345), TNF-α (Abcam, No. Ab6671), sialic acid (Abcam, No. Ab83375), CRP (Cayman Chemical, No. 10011236), haptoglobin (Abcam, No. Ab108856) and fibrinogen (AssayMax) were measured as recommended by producer Companies. The serum concentrations of triglycerides and cholesterol were assessed by commercial kits (Pars Azmoon; Iran).
Sperm parameters
At the end of the trial, following euthanasia, the right vas deferens were gathered. Sperm were collected by one syringe and needle using internal rinsing with 1.0ml of modified HTF medium (Human Tubal Fluid, Irvine Scientific) at 34°C. A Makler counting chamber heated up to 34°C was included with a small aliquot of sperm solution. Assessment of sperm motility was conducted by one person in all the trials and calculated by visual estimation (100 spermatozoa per animal, in duplicate) under a phase-contrast microscope (Leica DMLS) in 200X magnification. Spermatozoa were grouped as immotile, motile, lacking in progression, and motile, containing progressive movement. Sperm were also removed from the left vas deferens using internal rinsing with 1.0ml of saline formula by a syringe and needle. To evaluate the sperm morphology, smears were made histological slides left to dry for 90 minutes and observed in a phase-contrast microscope (400×magnification) [18], and 200 spermatozoa were assessed per rat. Morphological abnormalities were classified into two general categories, as reported by others [19].
Statistical Analysis
As the data was normal, all the analyses were conducted using the T-test, and correlation was conducted using Pearson correlation in SPSS 20 software. Figures were illustrated using Graph Pad Prism.
Findings
Body weight
Results in Figure 1 showed that experimental treatments did not influence body weight on day 1 (p>0.05). Still, body weight was significantly higher in rats fed high-fat diets compared to the control group on days 42 and 84 (p<0.05).

Figure 1. Effects of obesity on body weight on the different days in the rats
Lipid profile
The serum concentrations of cholesterol and triglycerides were significantly higher in obese rats compared to the control group (p<0.05; Figure 2).

Figure 2. Effects of obesity on the serum concentrations of cholesterol and triglycerides in the rats
Sperm quality
Our findings for sperm quality are shown in Figure 3. Results showed that progressive motility was significantly higher in the control group compared to obese rats (p<0.05). The non-progressive motility and immotility were significantly higher in obese rats compared to control groups (p<0.05).

Figure 3. Effects of obesity on sperm quality in rats
Inflammatory factors
The effects of obesity on inflammatory factors are shown in Table 1.
Table 1. Effects of experimental treatments on inflammatory markers (all significant at 0.05)

IL-1β, IL-6, IL-3, TNF-α, Sialic acid, CRP, Haptoglobin, and Fibrinogen were significantly higher in obese rats than in control rats (p<0.05). There was a negative correlation between progressive motility and a positive correlation between non-progressive motility and immotile with inflammatory factors (p<0.05).
Discussion
The diets used for obesity were efficient in promoting obesity, as highlighted by enhanced body weight. Increased body weight could be attributed to a hyperlipidemic diet. It has been accepted that consumption of high-fat diets raises oxidation. Increased oxidation enhances lipid deposition, i.e., lipid profile, which increases body weight [20, 21]. Our findings showed an increased lipid profile in obese rats, which confirms obesity. Increased oxidation during obesity could be attributed to oxidation, which increases lipolysis and lipid profile. Unfortunately, we did not evaluate oxidation parameters, but our findings for lipid parameters confirm obesity.
With regards to sperm quality, it was negatively influenced by experimental treatments. Sperm motility has been known as one of the most common variables used to assess sperm quality [22-24]. Sperm motility is obtained during sperm transition by the epididymal duct [25-29]. Previous studies have shown decreased motile sperm without changes in testosterone levels [30]. Morphological changes in obese animals show a high probability that obesity adversely influences spermatogenesis. It has been accepted that spermatogenesis is influenced in males with extreme obesity [31]. Our findings confirmed the adverse effects of obesity on sperm quality.
Increased inflammatory markers were observed in obese rats. Adipose tissue produces TNF-α and interleukins that are essential to energy regulation.
The ROS formed during inflammation is well thought-out as a factor, and amplified mitochondrial ROS production may have a significant role in the pathogenesis of obesity [32]. Metabolic inflammation is a major factor of obesity [33], and inflammatory signaling could considerably influence lipid metabolism in the liver [34]. TNF-α initiates the inflammation process, and then IL-1β, another pro-inflammatory cytokine, calls neutrophils into the region of infection [14]. CRP increases inflammatory responses [15]. Sialic acid and Hp increase during inflammation [16, 17]. We observed a relationship between inflammatory markers and sperm quality. Inflammation has been reported as a natural host response against microbial attack or tissue damage that restores tissue vasculature and functions [7, 8]. Inflammation increased spermatogenic arrest and inhibited processes of sperm maturation [4]. Inflammation specifically influences spermatocytes and spermatids but not spermatogonia [9]. So far, studies have not investigated the relationship between these factors and sperm quality. We could not find any study showing the relation between sperm quality and pro-inflammatory factors.
Conclusion
There is a positive relationship between obesity and lipid profile. Results also showed increased serum concentrations of IL-1β, IL-6, IL-3, TNF-α, sialic acid, CRP, haptoglobin, and fibrinogen in obese rats. Increased factors are correlated with decreased sperm quality.
Acknowledgments: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Bays H, Blonde L, Rosenson R. Adiposopathy: How do diet, exercise and weight loss drug therapies improve metabolic disease in overweight patients?. Exper Rev Cardiovasc Therap. 2009;4(6):871-95. [Link] [DOI:10.1586/14779072.4.6.871]
2. Kalaivani A, Uddandrao S, Parim B, Ganapathy S, Sushma NPR, Kancharla C, et al. Reversal of high fat diet-induced obesity through modulating lipid metabolic enzymes and inflammatory markers expressions in rats, Arch Physiol Biochem. 2018;125(3):28-34. [Link] [DOI:10.1080/13813455.2018.1452036]
3. Smit M, Romijn JC, Wildhagen MF, Weber RF, Dohle GR. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril. 2010;94(5):48-52. [Link] [DOI:10.1016/j.fertnstert.2009.10.030]
4. Adewoyin M, Ibrahim M, Roszaman R, LokmanMd Isa M, Mat Alewi NA, Abdul Rafa AA, et al. Male infertility: The effect of natural antioxidants and phytocompounds on seminal oxidative stress. Disease. 2017;5:9. [Link] [DOI:10.3390/diseases5010009]
5. Kashou AH, Plessis SS, Agarwal A. The Role of Obesity in ROS Generation and Male Infertility. In Studies on Men's Health and Fertility. New York: Humana Press; 2012. [Link] [DOI:10.1007/978-1-61779-776-7_26]
6. D'agata R, Vicari E, Moncada ML, Sidoti G, Calogero AE, Fornito MC, Polosa P. Generation of reactive oxygen species in subgroups of infertile men. Int J Androl. 1990;13:344-51. [Link] [DOI:10.1111/j.1365-2605.1990.tb01042.x]
7. Kvietys PR, Granger DN. Role of reactive oxygen and nitrogen species in the vascular responses to inflammation. Free Radic Biol Med. 2012;52:556-92. [Link] [DOI:10.1016/j.freeradbiomed.2011.11.002]
8. Adewoyin M, Mohsin SMN, Arulselvan P, Hussein MZ, Fakurazi S. Enhanced anti-inflammatorypotential of cinnamate-zinc layered hydroxide in lipopolysaccharide-stimulated RAW264.7 macrophages. Drug Des Dev Ther. 2015;9:2475-84. [Link] [DOI:10.2147/DDDT.S72716]
9. Liew SH, Meachem SJ, Hedger MPA. Stereological analysis of the response of spermatogenesis toan acute inflammatory episode in adult rats. J Androl. 2007;28:176-85. [Link] [DOI:10.2164/jandrol.106.000752]
10. Sebastian SM, Selvaraj S, Aruldhas MM, Govindarajulu P. Pattern of neutral and phospholipids in the semen of normospermic, oli¬gospermic and azoospermic men. J Reprod Fertil. 1987;79(2):373-8. [Link] [DOI:10.1530/jrf.0.0790373]
11. Tavilani H, Doosti M, Abdi K, Vaisiraygani A, Joshaghani HR. Decreased polyunsaturated and increased saturated fatty acid concentration in spermatozoa from asthenozoospermic males as compared with normozoospermic males. Andrologia. 2006;38(5):173-8. [Link] [DOI:10.1111/j.1439-0272.2006.00735.x]
12. Lenzi A, Picardo M, Gandini L, Dondero F. Lipids of the sperm plasma membrane: From polyunsaturated fatty acids considered as markers of sperm function to possible scavenger therapy. Hum Reprod Update. 1996;2(3):246-56. [Link] [DOI:10.1093/humupd/2.3.246]
13. Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110(6):731-6. [Link] [DOI:10.1172/JCI0216392]
14. Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478:1021-7. [Link] [DOI:10.1016/j.bbrc.2016.07.039]
15. Fujii T, Tabe Y, Yajima R, Tsutsumi S, Asao T, Kuwano H. Relationship between C-reactive protein levels and wound infections in elective colorectal surgery: C-Reactive protein as a predictor for incisional SSI. Hepato Gastroenterol. 2011;58:752-5. [Link]
16. Futamura G, Akihiro I, Chihara T,Kodama Y, Chihara T, Kaneko T, et al. Experimental research on stimulation of wound healing by n-3 fatty acids. Wounds. 2013;25(7):186-92. [Link]
17. Quaye IK. Haptoglobin, inflammation and disease. Transaction Royal Soci Tropic Med Hygiene. 2008;102(8):735-42. [Link] [DOI:10.1016/j.trstmh.2008.04.010]
18. Seed J, Chapi RE, Clegg ED, Dostal LA, Foote RE, Hurtt ME, et al. Methods for assessing sperm motility, morphology, and counts in the rat, rabbit, and dog: A consensus report. Reprod Toxicol. 1996;10(3):237-44. [Link] [DOI:10.1016/0890-6238(96)00028-7]
19. Filler R. Methods for evaluation of ratsepididymal sperm morphology. InMale reproductive toxicology. California: Academic Press; 1993. [Link] [DOI:10.1016/B978-0-12-461207-5.50025-0]
20. Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000;84(4):417-27. [Link] [DOI:10.1017/S0007114500001720]
21. Tentolouris N, Pavlatos S, Kokkinos A, Perrea D, Pagoni S, Katsilambros N. Diet-induced thermogenesis and substrate oxidation are not differentbetween lean and obese women after two different isocaloric meals,one rich in protein and one rich in fat. Metabolism. 2008;57(3):313-20. [Link] [DOI:10.1016/j.metabol.2007.10.004]
22. Mahadevan MM, Trounson AO. The influence of seminal characteristicson the sucess rate of human in vitro fertilization. Fertil Steril. 1984;42(3):400-5. [Link] [DOI:10.1016/S0015-0282(16)48080-5]
23. Bostofte E, Bagger P, Michael A, Stakemann G. Fertility prognosis forinfertile men from two different population evoluated by the Coxregression model. Fertil Steril. 1990;54(6):1100-6. [Link] [DOI:10.1016/S0015-0282(16)54012-6]
24. Barratt CL, Tomlinson MJ, Cooke ID. Prognostic significance ofcomputerised motility analysis for in vivo fertility. Fertil Steril. 1993;60(3):520-5. [Link] [DOI:10.1016/S0015-0282(16)56171-8]
25. Brooks DE. Epididymal functions and their hormonal regulation. Aust J Biol Sci. 1983,36(3):205-21. [Link] [DOI:10.1071/BI9830205]
26. Cooper TG. Epididymis. In encyclopedia of reproduction. Volume 2. California: Academic Press; 1998. [Link]
27. Jones RC. To store or mature spermatozoa? The primary role of theepididymis. Int J Androl. 1999;22(2):57-67. [Link] [DOI:10.1046/j.1365-2605.1999.00151.x]
28. Gatti JL, Castella S, Dacheux F, Ecruyd H, Métayer S, Thimon V, et al. Post-testicular sperm environment and fertility. AnimReprod Sci. 2004;82-83:321-39. [Link] [DOI:10.1016/j.anireprosci.2004.05.011]
29. Sullivan R, Saez F, Girouard J, Frenette G. Role of exossomes in spermmaturation during the transit along the male reproductive tract. Blood Cells Mol Dis. 2005;35(1):1-10. [Link] [DOI:10.1016/j.bcmd.2005.03.005]
30. Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesityin male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82(1):94-104. [Link] [DOI:10.1095/biolreprod.109.078915]
31. Klinefelter GR, Laskey JW, Perreault SD, Ferrel J, Jeffay S, Suarez J, et al. The ethane dimethanesulphonate-induced decrease in the fertilizingability of caudaepididymal sperm is independent of the testis. J Androl.1994;15(4):318-27. [Link] [DOI:10.1002/j.1939-4640.1994.tb00458.x]
32. Shen CL, Yeh J, Cao J. Green tea polyphenols mitigate bone loss of female rats in a chronic inflammation induced bone loss model. J NutrBiochem.2010;10:968-74. [Link] [DOI:10.1016/j.jnutbio.2009.08.002]
33. Solinas G, Karin M. Molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596-611. [Link] [DOI:10.1096/fj.09-151340]
34. Glass CK, Olefsky JM. Inflammation and lipid signalling in the etiology of insulin resistance. Cell metabolism. 2012;15:635-45. [Link] [DOI:10.1016/j.cmet.2012.04.001]









