GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 3 (2024)
GMJM 2024, 3(3): 93-97 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/02/15 | Accepted: 2024/07/28 | Published: 2024/09/12
Received: 2024/02/15 | Accepted: 2024/07/28 | Published: 2024/09/12
How to cite this article
Τsompos C, Panoulis C, Τοutouzas K, Triantafyllou A, Zografos G, Tsarea K, et al . Comparison of the Hypochloremic Effects of Erythropoietin and U-74389G. GMJM 2024; 3 (3) :93-97
URL: http://gmedicine.de/article-2-240-en.html
URL: http://gmedicine.de/article-2-240-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
C. Τsompos *1, C. Panoulis2, K. Τοutouzas3, A. Triantafyllou4, G.C. Zografos3, K. Tsarea5, M. Karamperi5, A. Papalois6
1- Department of Gynecology, General Hospital of Thessaloniki
2- Department of Obstetrics & Gynecology, Aretaieion Hospital, Athens University, Athens, Attiki, Hellas
3- Department of Surgery, Ippokrateion General Hospital, Athens University, Athens, Attiki, Hellas
4- Department of Biologic Chemistry, Athens University, Athens, Attiki, Hellas
5- Experimental Research Centre ELPEN Pharmaceuticals, S.A. Inc., Co., Pikermi, Attiki, Hellas
6- Educational and Research Center ELPEN European University Cyprus, School of Medicine, Cyprus
2- Department of Obstetrics & Gynecology, Aretaieion Hospital, Athens University, Athens, Attiki, Hellas
3- Department of Surgery, Ippokrateion General Hospital, Athens University, Athens, Attiki, Hellas
4- Department of Biologic Chemistry, Athens University, Athens, Attiki, Hellas
5- Experimental Research Centre ELPEN Pharmaceuticals, S.A. Inc., Co., Pikermi, Attiki, Hellas
6- Educational and Research Center ELPEN European University Cyprus, School of Medicine, Cyprus
Keywords:
| Abstract (HTML) (1303 Views)
Full-Text: (328 Views)
Introduction
The lazaroid U-74389G (21-[4-(2,6-di-1-pyrrolidinyl-4 -pyrimidinyl)-1 -piperazinyl]-pregnant-1,4,9(11)-triene-3,20-dione maleate salt), a novel antioxidant factor, may not be famous for its hypochloremic1 capacity. The ischemia-reperfusion (IR) type of experiments was noted in 19.15% of studies. A tissue protective feature of U-74389G was evident in these IR studies [1, 2]. The U-74389G is an antioxidant complex, which prevents lipid peroxidation, either iron-dependent or arachidonic acid-induced. After IR injury, animal kidney, liver, brain microvascular endothelial cell monolayers, and heart models were protected by U-74389G. U-74389G also attenuates the leukocytes, down-regulates the pro-inflammatory gene, treats the endotoxin shock, produces cytokine, enhances the mononuclear immunity, protects the endothelium, and presents antishock properties [3-7].
Even though erythropoietin (Epo) is not famous for its hypochloremic2 action, it can be used as a reference drug for comparison with U-74389G. Although Epo is met in over 31,147 published biomedical studies, only 3.66% of them negotiate the known type of IR experiments. Nevertheless, Epo, as a cytokine, is worthy of being studied for its effects on serum chloride (Cl) levels [8-15].
Although the most popular activities of neuroprotection and membrane-stabilization properties, it accumulates in the cell membrane, protecting vascular endothelium from peroxidative damage, but hardly penetrates the blood-brain barrier. It elicits a beneficial effect in ototoxicity and Duchenne muscular dystrophy. It increases γgt, superoxide dismutase (SOD) and glutathione (GSH) levels in oxygen-exposed cells. It treats septic states and acts as an immunosuppressant in flap survival. It prevents learning impairments and delays the early synaptic transmission decay during hypoxia, improving the energetic state of neurons. It shows antiproliferative properties on brain cancer cells and is considered a new promising anti-inflammatory drug for treating reperfusion syndrome in IR injuries [16, 17].
This experimental work compared the impact of the above drugs on a rat-induced IR protocol. The drugs were tested by calculating the serum Cl level alterations.
Materials and Methods
The preliminary references mention the vet licenses under 3693/12-11- 2010 & 14/10-1-2012 numbers, the granting company, and the experiment location [16].
The human-animal care of Albino female Wistar rats, the 7 days pre-experimental ad libitum diet, the non-stop intra-experimental anesthesiologic techniques, the audiometry, the electrocardiogram, the oxygen supply, and post-experimental euthanasia are also described in preliminary references. Rats were 16–18 weeks old. They were randomly assigned to six groups (each n=10). The stage of 45 min hypoxia was common for all 6 groups. Afterward, reperfusion of 60min was followed in group A; reperfusion of 120min in group B; immediate Epo intravenous (IV) administration and reperfusion of 60min in group C; immediate Epo IV administration and reperfusion of 120min in group D; immediate U-74389G IV administration and reperfusion of 60min in group E; and immediate U-74389G IV administration and reperfusion of 120min in Group F. The dose height assessment for both drugs was described in preliminary studies as 10mg/kg body mass.
Ischemia was caused by laparotomic clamping of the inferior aorta over renal arteries with forceps for 45 min. The clamp removal restored the inferior aorta patency and reperfusion. After excluding the blood flow, the IR protocol was applied, as described above, to each experimental group. The drugs were administered at the time of reperfusion through an inferior vena cava catheter. The Cl levels were determined at 60th min of reperfusion (for A, C, and E groups) and at 120th min of reperfusion (for B, D, and F groups). Also, no relation was raised between Cl values and animals’ mass (p=0.2175).
Findings
Table 1 presents the hypochloremic influence of erythropoietin and U-74389G regarding reperfusion time.
Table 1. The percentage of hypochloremic influence of erythropoietin and U-74389G according to reperfusion time
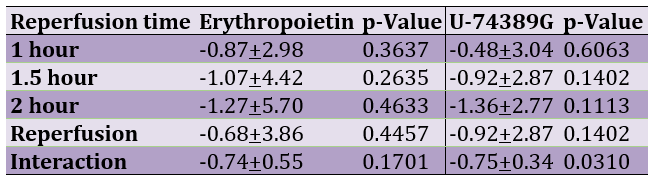
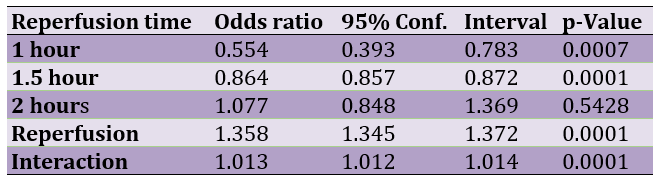
The successive application of chi-square tests revealed that U-74389G caused hypochloremia by 0.554-fold [0.393-0.783] less than Epo at 1h (p=0.0007), by 0.864-fold [0.857-0.872] less than Epo at 1.5h (p=0.0001), by 1.077-fold [0.848-1.369] more than Epo at 2h (p=0.5428), by 1.358-fold [1.345-1.372] more (p=0.0001) without drugs and by 1.0128-fold [1.012-1.013] more than Epo whether all variables have been considered (p=0.0001; Table 2).
Table 2. The U-74389G/erythropoietin hypochloremic efficacies after chi-square tests application
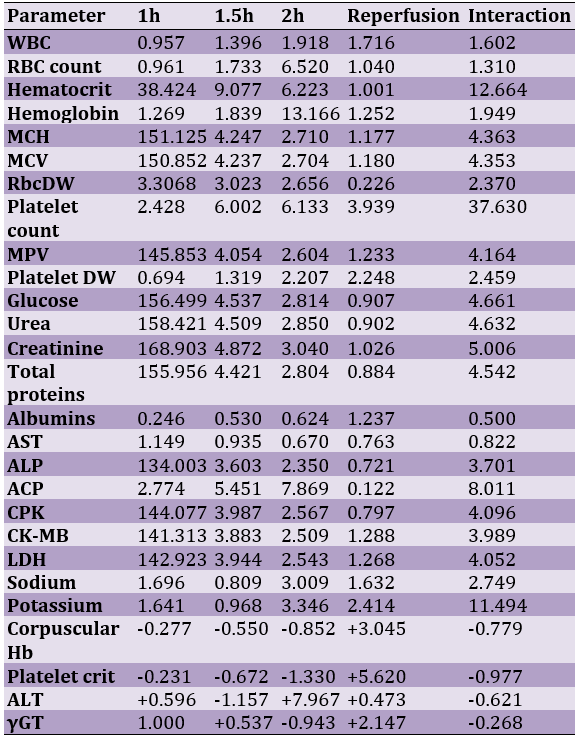
A meta-analysis of these ratios from the same experiment for 27 other series parameters provided comparable results (Table 3).
Table 3. A U-74389G/erythropoietin efficacies ratios meta-analysis (all were significant) on 27 hematologic variables (23 variables with balancing efficacies and 4 variables with opposite efficacies)
Discussion
The preliminary one was the unique available study investigating the hyperkalemic effect of U-74389G [1]. The same authors confirmed [2] the short-term hypochloremic effect of Epo preparations in non-iron-deficient individuals. Román-Anguiano et al. have shown [3] that NO inhibits the activity of caspases and calpains through S-nitrosylation of a cysteine located in their catalytic site since the independent cGMP pathway involves post-translational modification of proteins by S-nitrosylation. Infarct size was measured with 2,3,5-triphenyl tetrazolium chloride stain. S-nitrosylation of caspase-3 and calpain-1 was evaluated by labeling S-nitrosylated cysteines. Their results showed that both Prolame and SNAP increased NO content and improved functional recovery in post-ischemic hearts. Liu et al. showed [4] that mangiferin (MAF) could significantly reduce myocardial injury, inhibit myocardial oxidative stress and proinflammatory cytokines, and resume the ST segment after triphenyl tetrazolium chloride (TTC) staining and pathological analysis in H/R-induced rats H9c2 cells. Wang et al. suggested [5] that Wnt/β-catenin signaling is correlated with intermedin-induced angiogenesis and neovascularization in an in vitro model established by adding CoCl2 HUVECs. Shu et al. suggested [6] that troxerutin decreased neonatal rat cardiomyocyte apoptosis and alleviated myocardial I/R injury in rats via inhibition or downregulating miR-146a-5p in vivo. Infarct size was examined by 2,3,5-triphenyl tetrazolium chloride staining. Yin et al. summarized [7] that astragaloside IV attenuates myocardial I/R injury via inhibition of CaSR/ERK1/2 and the related apoptotic signaling pathways after MI/R injury by co-treatment with a calcium-sensing receptor CaSR agonist, gadolinium chloride (GdCl3) or a specific extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor U0126 in rats. Lou et al. demonstrated [8] that transforming growth beta (TGF-β) signaling activation regulates NADPH oxidase 2/NADPH oxidase 4 expression and exacerbates cerebral I/R injury in PC-12 cells. Rao et al. revealed [9] that special AT-rich sequence binding protein 1 (binds to nuclear matrix/scaffold-associating DNA) (SATB1) is a tumor promoter that participates in cancer cell migration and invasion. H/R-treated trophoblasts with lower SATB1 levels exhibited weaker invasive and growth capacities, whereas up-regulation of the SATB1 level with recombinant SATB1 restored these impairments. Moreover, the elevated concentration of SATB1 also increased the expression of β-catenin, which is involved in human placental trophoblast invasion, and differentiation is downregulated in PE. However, a specific activator, lithium chloride (LiCl), increased β-catenin expression but had no evident influence on SATB1 expression. Together, these data show that SATB1 expression in the human placenta is affected by oxidative stress and might regulate the migration and invasion of trophoblasts via β-catenin signaling. Mohamed et al. isolated [10] cardiomyocytes from newborn rats (0-2 days), and hypoxia was induced by using cobalt chloride (CoCl2). Their findings showed that ursodeoxycholic acid (UDCA) counteracted the effects of CoCl2 on cell viability, beating frequency, HIF-1α, and p53 protein expression. Furthermore, they observed that UDCA protects cardiomyocytes (CMs) against CoCl2-induced [Ca2+]i dynamic alteration. Pharmacological inhibition of the Gαi -sensitive receptor did not abolish the cardioprotection of UDCA against CoCl2 detrimental effects, except for cell viability and [Ca2+]i. Pertussis toxin partially inhibits UDCA protection against CoCl2 effects on CM cell viability. Interestingly, PTX fully inhibits UDCA cardioprotection on CoCl2-induced [Ca2+]i dynamic changes. They concluded that UDCA cardioprotection against CoCl2-induced hypoxia is similar to FTY720, and the Gαi does not fully mediate its actions -coupled protein-sensitive pathways. The current data generated were the first to show that UDCA can inhibit the activation of HIF-1α and p53 protein during CoCl2-induced hypoxia in cardiomyocytes. Qiu et al. acknowledged [11] that anion exchanger 3 (AE3) is crucial in maintaining intracellular chloride homeostasis by facilitating the reversible electroneutral exchange of Cl for HCO3 across the plasma membrane. Their previous studies reported that sasanqua saponin (SQS) could inhibit hypoxia/reoxygenation (H/R) induced elevation of intracellular Cl concentration ([Cl]i) and elicit cardioprotection by favoring Cl/HCO3 exchange of AE3. Additionally, both inhibition of PKCε by εV12 and S67A mutation of AE3 eradicated the SQS-induced increase of AE3 activity, reversed the inhibitory effect of SQS on H/R induced elevation of [Cl]i, Ca2+ overload and generation of reactive oxygen species and eliminated SQS induced cardioprotection. They concluded that PKCε dependent phosphorylation of serine 67 on AE3 might be responsible for increased Cl/HCO3 exchange of AE3 and intracellular chloride efflux by SQS in H9c2 cells. Zhang et al. found that luteolin (Lut) ameliorated [12] myocardial ischemia-reperfusion injury and H/R as evidenced by triphenyl tetrazolium chloride (TTC) staining and MTT assay, respectively. Lut also inhibited the upregulations of inflammasome components, such as NOD-like receptor 3(NLRP3), apoptosis-associated speck-like protein containing CARD(ASC) in I/R-induced rats and H/R-induced H9c2 cells. Zhang XG et al. demonstrated [13] that the isotonic substitution of extracellular chloride by gluconate (extracellular Cl--free) elicits cardioprotection by attenuating I/R-induced elevation of intracellular chloride ion concentration ([Cl-]i). The results showed that extracellular Cl--free attenuated H/R-induced the elevation of [Cl-]i, accompanied by increased cell viability and reduced lactate dehydrogenase release. Moreover, extracellular Cl--free inhibited mPTP opening and improved mitochondria function, as indicated by preserved mitochondrial membrane potential and respiratory chain complex activities, decreased mitochondrial reactive oxygen species generation, and increased ATP content. Intriguingly, pharmacologically, opening of the mPTP with Atr attenuated all the protective effects caused by extracellular Cl--free, including suppression of mPTP opening, maintenance of mitochondrial membrane potential, and subsequent improvement of mitochondrial function. These results indicated that extracellular Cl--free protects mitochondria from H/R injury in H9c2 cells, and inhibition of mPTP opening is crucial in mediating extracellular Cl--free cardioprotection. Li et al. reported [14] that sasanqua saponin (SQS) elicits cardioprotection by suppressing H/R-induced elevation of intracellular chloride ion concentration ([Cl-]i). The increased [Cl-]i modulates the mitochondrial permeability transition pore (mPTP). The results showed that SQS attenuated H/R-induced the elevation of [Cl-]i, accompanied by a reduction of lactate dehydrogenase release and an increase of cell viability. Interestingly, the extracellular Cl--free condition created by replacing Cl- with equimolar gluconate resulted in a decrease in [Cl-]i and induced protective effects similar to SQS preconditioning, whereas pharmacologically opening of the mPTP with ATR abolished all the protective effects induced by SQS or Cl--free, including suppression of mPTP opening, maintenance of mitochondrial membrane potential and subsequent improvement of mitochondrial function. The above results allow us to conclude that SQS-induced cardioprotection may be mediated by preserving the mitochondrial function by preventing mPTP opening via inhibition of H/R-induced elevation of [Cl-]i. Xia et al. reported that volume-sensitive outwardly rectifying (VSOR) Cl- channel-activated by reactive oxygen species (ROS) contributes [15] to cell apoptotic volume decrease, playing an incipient incident of cellular apoptosis in autophagy-related cell death. Interestingly, VSOR Cl- channel-blocked by VSOR Cl- channel blocker (DCPIB) could stably maintain the cell volume, intracellular pH, abundant lysosome-associated membrane protein-2 (LAMP2), and autophagic intensity regardless of ROS intension derived from reoxygenation injury or adding H2O2. These results demonstrate that VSOR Cl- channel-activated is a pivotal event to trigger autophagy-related death, revealing a novel therapeutic target to decrease myocardial I/R injury in rats.
Conclusion
The anti-oxidant agent U-74389G has a 1.013-fold [1.011746-1.01378] more hypochloremic effect than Epo.
Acknowledgments: None declared by the authors.
Ethical Permissions: We followed all applicable international, national, and/or institutional guidelines for the care and use of animals.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
The lazaroid U-74389G (21-[4-(2,6-di-1-pyrrolidinyl-4 -pyrimidinyl)-1 -piperazinyl]-pregnant-1,4,9(11)-triene-3,20-dione maleate salt), a novel antioxidant factor, may not be famous for its hypochloremic1 capacity. The ischemia-reperfusion (IR) type of experiments was noted in 19.15% of studies. A tissue protective feature of U-74389G was evident in these IR studies [1, 2]. The U-74389G is an antioxidant complex, which prevents lipid peroxidation, either iron-dependent or arachidonic acid-induced. After IR injury, animal kidney, liver, brain microvascular endothelial cell monolayers, and heart models were protected by U-74389G. U-74389G also attenuates the leukocytes, down-regulates the pro-inflammatory gene, treats the endotoxin shock, produces cytokine, enhances the mononuclear immunity, protects the endothelium, and presents antishock properties [3-7].
Even though erythropoietin (Epo) is not famous for its hypochloremic2 action, it can be used as a reference drug for comparison with U-74389G. Although Epo is met in over 31,147 published biomedical studies, only 3.66% of them negotiate the known type of IR experiments. Nevertheless, Epo, as a cytokine, is worthy of being studied for its effects on serum chloride (Cl) levels [8-15].
Although the most popular activities of neuroprotection and membrane-stabilization properties, it accumulates in the cell membrane, protecting vascular endothelium from peroxidative damage, but hardly penetrates the blood-brain barrier. It elicits a beneficial effect in ototoxicity and Duchenne muscular dystrophy. It increases γgt, superoxide dismutase (SOD) and glutathione (GSH) levels in oxygen-exposed cells. It treats septic states and acts as an immunosuppressant in flap survival. It prevents learning impairments and delays the early synaptic transmission decay during hypoxia, improving the energetic state of neurons. It shows antiproliferative properties on brain cancer cells and is considered a new promising anti-inflammatory drug for treating reperfusion syndrome in IR injuries [16, 17].
This experimental work compared the impact of the above drugs on a rat-induced IR protocol. The drugs were tested by calculating the serum Cl level alterations.
Materials and Methods
The preliminary references mention the vet licenses under 3693/12-11- 2010 & 14/10-1-2012 numbers, the granting company, and the experiment location [16].
The human-animal care of Albino female Wistar rats, the 7 days pre-experimental ad libitum diet, the non-stop intra-experimental anesthesiologic techniques, the audiometry, the electrocardiogram, the oxygen supply, and post-experimental euthanasia are also described in preliminary references. Rats were 16–18 weeks old. They were randomly assigned to six groups (each n=10). The stage of 45 min hypoxia was common for all 6 groups. Afterward, reperfusion of 60min was followed in group A; reperfusion of 120min in group B; immediate Epo intravenous (IV) administration and reperfusion of 60min in group C; immediate Epo IV administration and reperfusion of 120min in group D; immediate U-74389G IV administration and reperfusion of 60min in group E; and immediate U-74389G IV administration and reperfusion of 120min in Group F. The dose height assessment for both drugs was described in preliminary studies as 10mg/kg body mass.
Ischemia was caused by laparotomic clamping of the inferior aorta over renal arteries with forceps for 45 min. The clamp removal restored the inferior aorta patency and reperfusion. After excluding the blood flow, the IR protocol was applied, as described above, to each experimental group. The drugs were administered at the time of reperfusion through an inferior vena cava catheter. The Cl levels were determined at 60th min of reperfusion (for A, C, and E groups) and at 120th min of reperfusion (for B, D, and F groups). Also, no relation was raised between Cl values and animals’ mass (p=0.2175).
Findings
Table 1 presents the hypochloremic influence of erythropoietin and U-74389G regarding reperfusion time.
Table 1. The percentage of hypochloremic influence of erythropoietin and U-74389G according to reperfusion time

The successive application of chi-square tests revealed that U-74389G caused hypochloremia by 0.554-fold [0.393-0.783] less than Epo at 1h (p=0.0007), by 0.864-fold [0.857-0.872] less than Epo at 1.5h (p=0.0001), by 1.077-fold [0.848-1.369] more than Epo at 2h (p=0.5428), by 1.358-fold [1.345-1.372] more (p=0.0001) without drugs and by 1.0128-fold [1.012-1.013] more than Epo whether all variables have been considered (p=0.0001; Table 2).
Table 2. The U-74389G/erythropoietin hypochloremic efficacies after chi-square tests application

A meta-analysis of these ratios from the same experiment for 27 other series parameters provided comparable results (Table 3).
Table 3. A U-74389G/erythropoietin efficacies ratios meta-analysis (all were significant) on 27 hematologic variables (23 variables with balancing efficacies and 4 variables with opposite efficacies)

Discussion
The preliminary one was the unique available study investigating the hyperkalemic effect of U-74389G [1]. The same authors confirmed [2] the short-term hypochloremic effect of Epo preparations in non-iron-deficient individuals. Román-Anguiano et al. have shown [3] that NO inhibits the activity of caspases and calpains through S-nitrosylation of a cysteine located in their catalytic site since the independent cGMP pathway involves post-translational modification of proteins by S-nitrosylation. Infarct size was measured with 2,3,5-triphenyl tetrazolium chloride stain. S-nitrosylation of caspase-3 and calpain-1 was evaluated by labeling S-nitrosylated cysteines. Their results showed that both Prolame and SNAP increased NO content and improved functional recovery in post-ischemic hearts. Liu et al. showed [4] that mangiferin (MAF) could significantly reduce myocardial injury, inhibit myocardial oxidative stress and proinflammatory cytokines, and resume the ST segment after triphenyl tetrazolium chloride (TTC) staining and pathological analysis in H/R-induced rats H9c2 cells. Wang et al. suggested [5] that Wnt/β-catenin signaling is correlated with intermedin-induced angiogenesis and neovascularization in an in vitro model established by adding CoCl2 HUVECs. Shu et al. suggested [6] that troxerutin decreased neonatal rat cardiomyocyte apoptosis and alleviated myocardial I/R injury in rats via inhibition or downregulating miR-146a-5p in vivo. Infarct size was examined by 2,3,5-triphenyl tetrazolium chloride staining. Yin et al. summarized [7] that astragaloside IV attenuates myocardial I/R injury via inhibition of CaSR/ERK1/2 and the related apoptotic signaling pathways after MI/R injury by co-treatment with a calcium-sensing receptor CaSR agonist, gadolinium chloride (GdCl3) or a specific extracellular signal-regulated kinase 1/2 (ERK1/2) inhibitor U0126 in rats. Lou et al. demonstrated [8] that transforming growth beta (TGF-β) signaling activation regulates NADPH oxidase 2/NADPH oxidase 4 expression and exacerbates cerebral I/R injury in PC-12 cells. Rao et al. revealed [9] that special AT-rich sequence binding protein 1 (binds to nuclear matrix/scaffold-associating DNA) (SATB1) is a tumor promoter that participates in cancer cell migration and invasion. H/R-treated trophoblasts with lower SATB1 levels exhibited weaker invasive and growth capacities, whereas up-regulation of the SATB1 level with recombinant SATB1 restored these impairments. Moreover, the elevated concentration of SATB1 also increased the expression of β-catenin, which is involved in human placental trophoblast invasion, and differentiation is downregulated in PE. However, a specific activator, lithium chloride (LiCl), increased β-catenin expression but had no evident influence on SATB1 expression. Together, these data show that SATB1 expression in the human placenta is affected by oxidative stress and might regulate the migration and invasion of trophoblasts via β-catenin signaling. Mohamed et al. isolated [10] cardiomyocytes from newborn rats (0-2 days), and hypoxia was induced by using cobalt chloride (CoCl2). Their findings showed that ursodeoxycholic acid (UDCA) counteracted the effects of CoCl2 on cell viability, beating frequency, HIF-1α, and p53 protein expression. Furthermore, they observed that UDCA protects cardiomyocytes (CMs) against CoCl2-induced [Ca2+]i dynamic alteration. Pharmacological inhibition of the Gαi -sensitive receptor did not abolish the cardioprotection of UDCA against CoCl2 detrimental effects, except for cell viability and [Ca2+]i. Pertussis toxin partially inhibits UDCA protection against CoCl2 effects on CM cell viability. Interestingly, PTX fully inhibits UDCA cardioprotection on CoCl2-induced [Ca2+]i dynamic changes. They concluded that UDCA cardioprotection against CoCl2-induced hypoxia is similar to FTY720, and the Gαi does not fully mediate its actions -coupled protein-sensitive pathways. The current data generated were the first to show that UDCA can inhibit the activation of HIF-1α and p53 protein during CoCl2-induced hypoxia in cardiomyocytes. Qiu et al. acknowledged [11] that anion exchanger 3 (AE3) is crucial in maintaining intracellular chloride homeostasis by facilitating the reversible electroneutral exchange of Cl for HCO3 across the plasma membrane. Their previous studies reported that sasanqua saponin (SQS) could inhibit hypoxia/reoxygenation (H/R) induced elevation of intracellular Cl concentration ([Cl]i) and elicit cardioprotection by favoring Cl/HCO3 exchange of AE3. Additionally, both inhibition of PKCε by εV12 and S67A mutation of AE3 eradicated the SQS-induced increase of AE3 activity, reversed the inhibitory effect of SQS on H/R induced elevation of [Cl]i, Ca2+ overload and generation of reactive oxygen species and eliminated SQS induced cardioprotection. They concluded that PKCε dependent phosphorylation of serine 67 on AE3 might be responsible for increased Cl/HCO3 exchange of AE3 and intracellular chloride efflux by SQS in H9c2 cells. Zhang et al. found that luteolin (Lut) ameliorated [12] myocardial ischemia-reperfusion injury and H/R as evidenced by triphenyl tetrazolium chloride (TTC) staining and MTT assay, respectively. Lut also inhibited the upregulations of inflammasome components, such as NOD-like receptor 3(NLRP3), apoptosis-associated speck-like protein containing CARD(ASC) in I/R-induced rats and H/R-induced H9c2 cells. Zhang XG et al. demonstrated [13] that the isotonic substitution of extracellular chloride by gluconate (extracellular Cl--free) elicits cardioprotection by attenuating I/R-induced elevation of intracellular chloride ion concentration ([Cl-]i). The results showed that extracellular Cl--free attenuated H/R-induced the elevation of [Cl-]i, accompanied by increased cell viability and reduced lactate dehydrogenase release. Moreover, extracellular Cl--free inhibited mPTP opening and improved mitochondria function, as indicated by preserved mitochondrial membrane potential and respiratory chain complex activities, decreased mitochondrial reactive oxygen species generation, and increased ATP content. Intriguingly, pharmacologically, opening of the mPTP with Atr attenuated all the protective effects caused by extracellular Cl--free, including suppression of mPTP opening, maintenance of mitochondrial membrane potential, and subsequent improvement of mitochondrial function. These results indicated that extracellular Cl--free protects mitochondria from H/R injury in H9c2 cells, and inhibition of mPTP opening is crucial in mediating extracellular Cl--free cardioprotection. Li et al. reported [14] that sasanqua saponin (SQS) elicits cardioprotection by suppressing H/R-induced elevation of intracellular chloride ion concentration ([Cl-]i). The increased [Cl-]i modulates the mitochondrial permeability transition pore (mPTP). The results showed that SQS attenuated H/R-induced the elevation of [Cl-]i, accompanied by a reduction of lactate dehydrogenase release and an increase of cell viability. Interestingly, the extracellular Cl--free condition created by replacing Cl- with equimolar gluconate resulted in a decrease in [Cl-]i and induced protective effects similar to SQS preconditioning, whereas pharmacologically opening of the mPTP with ATR abolished all the protective effects induced by SQS or Cl--free, including suppression of mPTP opening, maintenance of mitochondrial membrane potential and subsequent improvement of mitochondrial function. The above results allow us to conclude that SQS-induced cardioprotection may be mediated by preserving the mitochondrial function by preventing mPTP opening via inhibition of H/R-induced elevation of [Cl-]i. Xia et al. reported that volume-sensitive outwardly rectifying (VSOR) Cl- channel-activated by reactive oxygen species (ROS) contributes [15] to cell apoptotic volume decrease, playing an incipient incident of cellular apoptosis in autophagy-related cell death. Interestingly, VSOR Cl- channel-blocked by VSOR Cl- channel blocker (DCPIB) could stably maintain the cell volume, intracellular pH, abundant lysosome-associated membrane protein-2 (LAMP2), and autophagic intensity regardless of ROS intension derived from reoxygenation injury or adding H2O2. These results demonstrate that VSOR Cl- channel-activated is a pivotal event to trigger autophagy-related death, revealing a novel therapeutic target to decrease myocardial I/R injury in rats.
Conclusion
The anti-oxidant agent U-74389G has a 1.013-fold [1.011746-1.01378] more hypochloremic effect than Epo.
Acknowledgments: None declared by the authors.
Ethical Permissions: We followed all applicable international, national, and/or institutional guidelines for the care and use of animals.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Τsompos C, Panoulis C, Τοutouzas K, Ζografos G, Papalois A. The effect of the antioxidant drug "U-74389G" on chloride during ischemia reperfusion injury in rats. Med Rev. 2014;50(2):40-4. [Link] [DOI:10.2174/1871523013666140804230111]
2. Tsompos C, Panoulis C, Toutouzas K, Triantafyllou A, Zografos G, Papalois A. The effect of erythropoietin on chloride levels during hypoxia reoxygenation injury in rats. Signa Vitae. 2017;13(2):97-101. [Link] [DOI:10.22514/SV132.112017.15]
3. Román-Anguiano NG, Correa F, Cano-Martínez A, de la Peña-Díaz A, Zazueta C. Cardioprotective effects of Prolame and SNAP are related with nitric oxide production and with diminution of caspases and calpain-1 activities in reperfused rat hearts. Peer J. 2019;29(7):e7348. [Link] [DOI:10.7717/peerj.7348]
4. Liu K, Wang F, Wang S, Li WN, Ye Q. Mangiferin attenuates myocardial ischemia-reperfusion injury via MAPK/Nrf-2/HO-1/NF-κB in vitro and in vivo. Oxid Med Cell Longev. 2019;13:7285434. [Link] [DOI:10.1155/2019/7285434]
5. Wang Y, Wu Z, Tian J, Mi Y, Ren X, Kang J, et al. Intermedin protects HUVECs from ischemia reperfusion injury via Wnt/β-catenin signaling pathway. Ren Fail. 2019;41(1):159-66. [Link] [DOI:10.1080/0886022X.2019.1587468]
6. Shu L, Zhang W, Huang G, Huang C, Zhu X, Su G, et al. Troxerutin attenuates myocardial cell apoptosis following myocardial ischemia-reperfusion injury through inhibition of miR-146a-5p expression. J Cell Physiol. 2019;234(6):9274-82. [Link] [DOI:10.1002/jcp.27607]
7. Yin B, Hou XW, Lu ML. Astragaloside IV attenuates myocardial ischemia/reperfusion injury in rats via inhibition of calcium-sensing receptor-mediated apoptotic signaling pathways. Acta Pharmacol Sin. 2019;40(5):599-607. [Link] [DOI:10.1038/s41401-018-0082-y]
8. Lou Z, Wang AP, Duan XM, Hu GH, Song GL, Zuo ML, et al. Upregulation of NOX2 and NOX4 mediated by TGF-β signaling pathway exacerbates cerebral ischemia/reperfusion oxidative stress injury. Cell Physiol Biochem. 2018;46(5):2103-13. [Link] [DOI:10.1159/000489450]
9. Rao H, Bai Y, Li Q, Zhuang B, Yuan Y, Liu Y. SATB1 downregulation induced by oxidative stress participates in trophoblast invasion by regulating β-catenin. Biol Reprod. 2018;98(6):810-20. [Link] [DOI:10.1093/biolre/ioy033]
10. Mohamed AS, Hanafi NI, Sheikh Abdul Kadir SH, Md Noor J, Abdul Hamid Hasani N, Rahim SA, et al. Ursodeoxycholic acid protects cardiomyocytes against cobalt chloride induced hypoxia by regulating transcriptional mediator of cells stress hypoxia inducible factor 1α and p53 protein. Cell Biochem Funct. 2017;35(7):453-63. [Link] [DOI:10.1002/cbf.3303]
11. Qiu LY, Duan GL, Yan YF, Li YY, Wang H, Xiao L, et al. Sasanquasaponin induces increase of Cl /HCO3 exchange of anion exchanger 3 and promotes intracellular Cl efflux in hypoxia/reoxygenation cardiomyocytes. Mol Med Rep. 2017;16(3):2953-61. [Link] [DOI:10.3892/mmr.2017.6882]
12. Zhang X, Du Q, Yang Y, Wang J, Dou S, Liu C, et al. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed Pharmacother. 2017;91:1042-52. [Link] [DOI:10.1016/j.biopha.2017.05.033]
13. Zhang XG, Zhao L, Zhang Y, Li YY, Wang H, Duan GL, et al. Extracellular Cl-free-induced cardioprotection against hypoxia/reoxygenation is associated with attenuation of mitochondrial permeability transition pore. Biomed Pharmacother. 2017;86:637-44. [Link] [DOI:10.1016/j.biopha.2016.12.048]
14. Li YY, Xiao L, Qiu LY, Yan YF, Wang H, Duan GL, et al. Sasanquasaponin-induced cardioprotection involves inhibition of mPTP opening via attenuating intracellular chloride accumulation. Fitoterapia. 2017;116:1-9. [Link] [DOI:10.1016/j.fitote.2016.11.003]
15. Xia Y, Liu Y, Xia T, Li X, Huo C, Jia X, et al. Activation of volume-sensitive Cl- channel mediates autophagy-related cell death in myocardial ischaemia/reperfusion injury. Oncotarget. 2016;7(26):39345-62. [Link] [DOI:10.18632/oncotarget.10050]
16. Τsompos C, Panoulis C, Τοutouzas K, Triantafyllou A, Ζografos CG, Tsarea K, et al. Comparison of the hyperkalemic effects of erythropoietin and U-74389G. Int J Womens Health Gynecol. 2019;1(2):107. [Link] [DOI:10.15226/2475-4714/2/2/00122]
17. Tsompos C, Panoulis C, Toutouzas K, Triantafyllou A, Zografos GC, Tsarea K, et al. Comparison of the hyponatremic effects of erythropoietin and U-74389G. Insight Biomed Res. 2019;3(1):52-5. [Link] [DOI:10.33805/2690-2613.101]









