GMJ Medicine
eISSN : 2626-3041
Volume 4, Issue 1 (2025)
GMJM 2025, 4(1): 25-29 |
Back to browse issues page
History
Received: 2024/06/25 | Accepted: 2024/12/7 | Published: 2025/01/20
Received: 2024/06/25 | Accepted: 2024/12/7 | Published: 2025/01/20
How to cite this article
Yousefi S, Nemati Karimooy F, Miyanbandi T, Esmaeilpour F. Effect of Dietary Supplementing with Resveratrol on the Antioxidant Status of Hypercholestrolemic Rats. GMJM 2025; 4 (1) :25-29
URL: http://gmedicine.de/article-2-246-en.html
URL: http://gmedicine.de/article-2-246-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Biochemistry, Faculty of Medicine, Semnan University of Medical Science, Semnan, Iran
2- Medical School, Mashhad University of Medical Sciences, Mashhad, Iran
3- School of Nursing & Midwifery, Neyshabur Branch, Islamic Azad University, Neyshabur, Iran
4- Georgian Center for Neuroscience Research, International Center for Intelligent Research, Tbilisi, Georgia
2- Medical School, Mashhad University of Medical Sciences, Mashhad, Iran
3- School of Nursing & Midwifery, Neyshabur Branch, Islamic Azad University, Neyshabur, Iran
4- Georgian Center for Neuroscience Research, International Center for Intelligent Research, Tbilisi, Georgia
Keywords:
| Abstract (HTML) (796 Views)
Full-Text: (148 Views)
Introduction
Hypercholesterolemia is known as a lipoprotein metabolic disorder which is associated with increased high serum low density lipoprotein (LDL) and serum cholesterol [1]. Hypercholesterolemia is a challenging issue for some societies and also health professionals that is mainly due to close correlation between cardiovascular diseases and lipid profile abnormalities [2].
Hypercholesterolemia usually leads to the nonalcoholic fatty liver disease (NAFLD) through accumulation of triglycerides and other fats in liver that causes liver failure and hepatocellular carcinoma [3, 4]. An important reason related to NAFLD is increased oxidative stress that may disturb desaturation activities [5, 6].
Oxidative stress is reported to have a strong relation with a broad range of pathologies such as inflammation, cancer, neurologic disorders and metabolic diseases like obesity and hypercholesterolemia [7, 8]. It has also showed a close correlation with cumulative damage resulting from reactive oxygen species (ROS) and reactive nitrogen species (RNS) that are neutralized via antioxidants pathways [9,10]. It has been reported that free radicals may negatively influence the cell’s survival against an oxidative damage to macromolecules [11, 12].
Therefore, damages to liver are supposed to be treated with antioxidant enzymes [13]. Oxidative damage is significantly increased with a decrease in the activity of antioxidant enzymes that clean the free radicals which are involved in the oxidative stress [14]. Superoxide dismutase (SOD) is one of the significant enzymatic antioxidant pathways against superoxide radical that inhibits liver toxicity due to oxidative stress [15]. Catalase (CAT) and glutathione peroxidase (GPx) convert H2O2 to water and provide protection against ROS [16]. Paraoxonase (PON1) is another antioxidant enzyme which is related to high-density lipoproteins (HDL) and detoxifies lipid peroxides. This enzyme is broadly distributed in some tissues including liver [17]. Sulfiredoxin-1 enzyme belongs to the family of oxidoreductases that catalyzes conversion of cysteine sulfinic acid into sulfenic acid in oxidized proteins and protects them from inactivation [18]. Glutamate-cysteine has significant importance in formation of glutathione that acts against a class of oxidative stress associated with many complications [19].
Today, natural agents have been used as protection against oxidative damages. Natural antioxidants are appropriate choices for mitigating the adverse effects of hypercholesterolemia in terms of antioxidant parameters. Resveratrol, as a phytoestrogen, is known to have antioxidant and anti-inflammatory properties and can be found in the different plant species. Beneficial effects of resveratrol on some disorders including type 2 diabetes, cardiovascular diseases, cancer and neurological disorders have been reported [20]. It is stated that resveratrol regulates energy consumption in animals with high-fat diet. Resveratrol can effectively reduce weight gain and intra-abdominal fat and improve the lipid profile in obese mice [21]. It seems that resveratrol may probably improve some biochemical and antioxidant parameters in rats with hypercholesterolemia. This study was therefore conducted to evaluate the beneficial effects of dietary supplementing with resveratrol on improving the antioxidant status in hypercholesterolemic rats.
Materials and Methods
Animals
Ninety female Wistar rats (weighting 80-100g and five weeks of age), were purchased from the Pasture Institute of Iran. Animals were hosted in laboratory conditions from 10 days prior to the trial. These conditions included standard temperature (22±1°C) and humidity (50-55%) and 12-hour light/dark cycles with free access to food and water. They were given a prepared food from Javaneh Khorasan Company in powder form. Animals were divided into 6 different groups (n=15) and were treated for 6 weeks continuously. Resveratrol was purchased from Sigma Aldrich Company and administered to rats in different doses.
The control group was given the basal diet merely whereas the positive control group (Control HC) had the hypercholestrol diet (basal diet plus 1% cholesterol and 0.5% cholic acid). Two experimental groups (2.5 RES and 5 RES) received resveratrol with doses of 2.5 and 5 mg/kg separately in addition to their basal diet. The other two test groups were given the HC diet while treated with 2.5 and 5 mg/kg of resveratrol (2.5 RES-HC and 5 RES-HC).
Chemical Analysis
In the end of the trial, rats were anaesthetized and blood samples were collected and centrifuged to prepare the serum plasma. Liver tissues were immediately extracted and kept in -80°C for future experiments. The plasma levels of total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL), malondialdehyde (MDA), and low density lipoprotein (LDL) were evaluated by available diagnostic kits (Pars Azmoon, Tehran, Iran). The levels of glutathione peroxidase (GPx), glutathione reductase (GR), paraoxonase-1 and sulfiredoxin-1 were assessed as it was previously reported by Al-Rejaie et al. [22]. The primers sequences were GPx, forward (5'- GGTGTTCCAGTGCGCAGAT-3') and reverse (5'-AGGGCTTCTATATCGGGTTCGA-3'); GR, forward (5'-TGAGCCGCCTGAACAACA-3') and reverse (5'-TTGCGTAGCCGTGGATGAC-3'); Paraoxonase-1, forward (5′- TGAGAGCTTCTATGCCACAAATG-3′) and reverse (5′-CCATGACAGGCCCAAGTACA-3′); Sulfiredoxin-1, forward (5′- AATCCCCAACCCCTGACTTT-3′) and reverse (5′- TGAACTGACCAGTGGAGACACAGT-3′).
Statistical Analysis
One way analysis of variance following by the Tukey-Kramer multiple comparison test was used to compare the data.
Findings
Biochemical parameters
The plasma concentrations of cholesterol, triglycerides, LDL-C and malondialdehyde were significantly higher and the level of HDL was significantly lower in hypercholesterolemic rats (p<0.05; Table 1).
Table 1. Effects of resveratrol on biochemical parameters in hypercholesterolemic rats
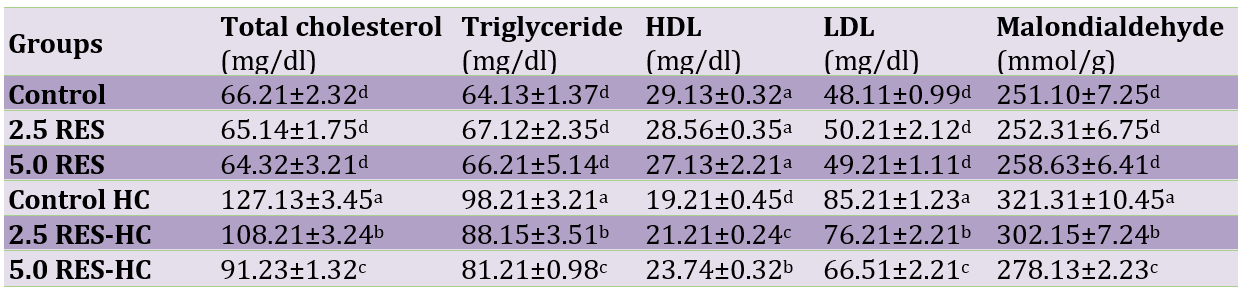
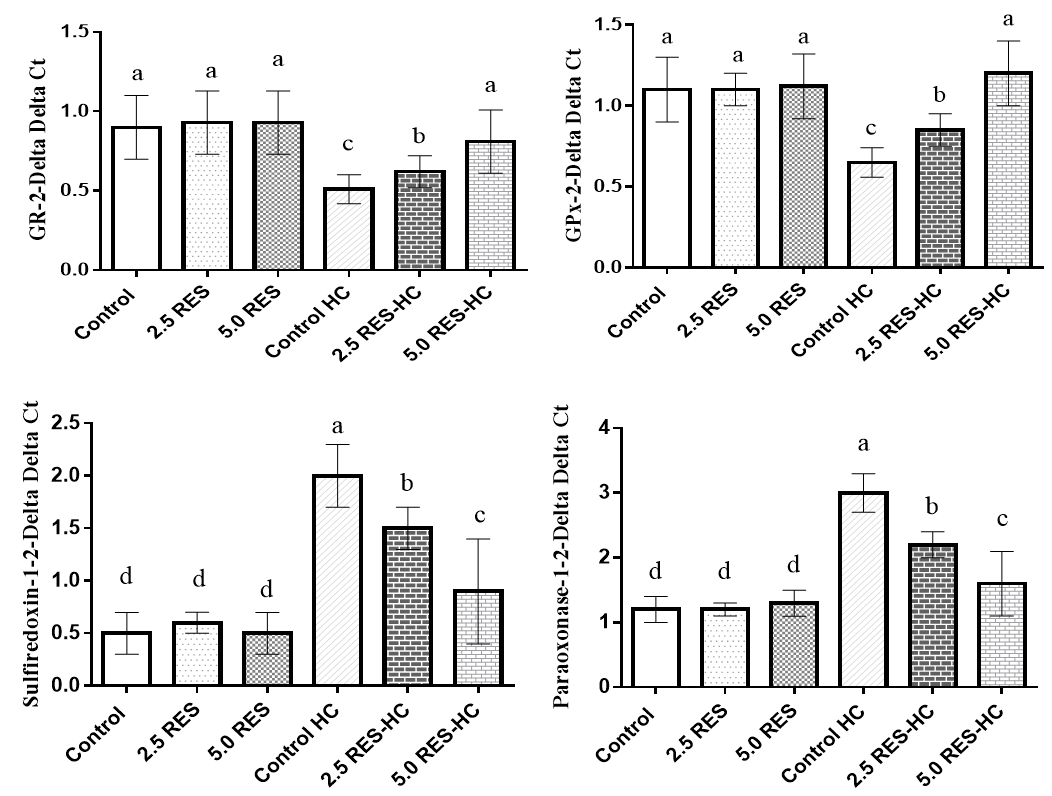
Figure 1. Effects of resveratrol on antioxidant status in hypercholesterolemic rats
Dietary supplementation with resveratrol, especially in higher level (5mg/kg) could decrease the levels of cholesterol, triglycerides, LDL-C and malondialdehyde and increase the HDL-C level (p<0.05). Dietary inclusion of resveratrol had no significant effects on blood parameters in healthy rats (p>0.05).
Antioxidant status
Effects of resveratrol on antioxidant status in hypercholesterolemic rats are depicted in figure 1. Hypercholesterolemic rats showed a decrease in GPx and GR and an increase in paraoxonase-1 and sulfiredoxin-1 in comparison to healthy control (p<0.05), but administration of resveratrol into diet could reverse these changes (p<0.05). There was no significant difference between treated and healthy rats in mentioned parameters (p>0.05).
Discussion
Obesity has been known as a risk factor for some diseases including cardiovascular and liver diseases [3, 4]. Hypercholesterolemic A suitable animal model for human obesity syndrome is a rat with hypercholesterolemia [23]. Our findings showed that lipid profile was changed in rats with hypercholestrolemia. Lipid profile changes have been known as a contributory factor in oxidative stress related to obesity that is caused by an increase in the production of ROS as well as a decrease in antioxidant enzymes [24]. ROSs and lipid peroxidation products disturb the respiratory chain in hepatocytes by oxidative damage to the mitochondrial DNA. Previous studies have reported a change in lipid profile of hypercholesterolemic rats [25]. High cholesterol diet may cause dyslipidemia syndrome and hyperlipidemia that is characterized by increased triglycerides in addition to decreased HDL-C [26]. Such results were also observed in the current study. Our findings showed that resveratrol could improve lipid profile. It has been previously reported that resveratrol was responsible for an improvement in serum lipids [27].
Resveratrol has also been reported to have antioxidant effects by reducing H2O2 and lipid peroxidation in the skin [28]. Previous studies have shown that treatment with resveratrol improves atherosclerosis through decreasing lipid drops in the intima of the aorta and also reducing vascular oxidative stress [29]. Our findings showed that hypercholesterolemia had elevated lipid peroxidation in hepatic tissue which is highlighted by increased plasma level of MDA. MDA increases the accumulation of H2O2 and finally promotes the lipid peroxidation. Our observations suggested that resveratrol reduces the level of MDA and improves the lipid profile that is probably as a result of decreased MDA.
Antioxidant system plays a significant role in the detoxification process in the liver. GPx is a selenoenzyme that catalyzes the reduction of hydrogen peroxide to H2O [30]. Down-regulation of GR provoke some responses that increases oxidative stress. Our findings showed that hypercholesterolemia increases the production of free radicals and also decreases the ability to detoxify ROS which leads to hepatocellular damage [31]. These findings show that resveratrol has antioxidant activity. Paraoxonase-1 is an enzyme with lactonase and esterase activities which is produced in the liver [32] and is known to have a role in regulating oxidative stress, fibrosis and hepatic cell apoptosis in chronic liver diseases [33]. Increased paraoxonase-1 expression develop the sensitivity to liver damage, chronic hepatitis and liver cirrhosis. Sulfiredoxin-1 is an antioxidant enzyme which contains a C-terminal cysteine residue and is essential for its antioxidant activity [34]. It is also known to play a role in cellular responses to oxidative stress through restoring the activity of over-oxidized peroxiredoxins [34]. These findings show importance of resveratrol for treating hepatitis and cirrhosis and reducing the oxidative stress.
Conclusion
Hypercholesterolemia causes imbalances in the lipid profile and antioxidant system but resveratrol in high doses reverses these adverse effects.
Acknowledgements: None declared by the authors.
Ethical Permission: Compliance with ethical guidelines Approval for this study was obtained from International Center for Intelligent Research Ethics Committee (Tbilisi, Georgia).
Conflicts of Interests: None declared by the authors.
Authors' Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: This study was supported by a grant from International Center for Intelligent Research (ICIR-2017-154789).
Hypercholesterolemia is known as a lipoprotein metabolic disorder which is associated with increased high serum low density lipoprotein (LDL) and serum cholesterol [1]. Hypercholesterolemia is a challenging issue for some societies and also health professionals that is mainly due to close correlation between cardiovascular diseases and lipid profile abnormalities [2].
Hypercholesterolemia usually leads to the nonalcoholic fatty liver disease (NAFLD) through accumulation of triglycerides and other fats in liver that causes liver failure and hepatocellular carcinoma [3, 4]. An important reason related to NAFLD is increased oxidative stress that may disturb desaturation activities [5, 6].
Oxidative stress is reported to have a strong relation with a broad range of pathologies such as inflammation, cancer, neurologic disorders and metabolic diseases like obesity and hypercholesterolemia [7, 8]. It has also showed a close correlation with cumulative damage resulting from reactive oxygen species (ROS) and reactive nitrogen species (RNS) that are neutralized via antioxidants pathways [9,10]. It has been reported that free radicals may negatively influence the cell’s survival against an oxidative damage to macromolecules [11, 12].
Therefore, damages to liver are supposed to be treated with antioxidant enzymes [13]. Oxidative damage is significantly increased with a decrease in the activity of antioxidant enzymes that clean the free radicals which are involved in the oxidative stress [14]. Superoxide dismutase (SOD) is one of the significant enzymatic antioxidant pathways against superoxide radical that inhibits liver toxicity due to oxidative stress [15]. Catalase (CAT) and glutathione peroxidase (GPx) convert H2O2 to water and provide protection against ROS [16]. Paraoxonase (PON1) is another antioxidant enzyme which is related to high-density lipoproteins (HDL) and detoxifies lipid peroxides. This enzyme is broadly distributed in some tissues including liver [17]. Sulfiredoxin-1 enzyme belongs to the family of oxidoreductases that catalyzes conversion of cysteine sulfinic acid into sulfenic acid in oxidized proteins and protects them from inactivation [18]. Glutamate-cysteine has significant importance in formation of glutathione that acts against a class of oxidative stress associated with many complications [19].
Today, natural agents have been used as protection against oxidative damages. Natural antioxidants are appropriate choices for mitigating the adverse effects of hypercholesterolemia in terms of antioxidant parameters. Resveratrol, as a phytoestrogen, is known to have antioxidant and anti-inflammatory properties and can be found in the different plant species. Beneficial effects of resveratrol on some disorders including type 2 diabetes, cardiovascular diseases, cancer and neurological disorders have been reported [20]. It is stated that resveratrol regulates energy consumption in animals with high-fat diet. Resveratrol can effectively reduce weight gain and intra-abdominal fat and improve the lipid profile in obese mice [21]. It seems that resveratrol may probably improve some biochemical and antioxidant parameters in rats with hypercholesterolemia. This study was therefore conducted to evaluate the beneficial effects of dietary supplementing with resveratrol on improving the antioxidant status in hypercholesterolemic rats.
Materials and Methods
Animals
Ninety female Wistar rats (weighting 80-100g and five weeks of age), were purchased from the Pasture Institute of Iran. Animals were hosted in laboratory conditions from 10 days prior to the trial. These conditions included standard temperature (22±1°C) and humidity (50-55%) and 12-hour light/dark cycles with free access to food and water. They were given a prepared food from Javaneh Khorasan Company in powder form. Animals were divided into 6 different groups (n=15) and were treated for 6 weeks continuously. Resveratrol was purchased from Sigma Aldrich Company and administered to rats in different doses.
The control group was given the basal diet merely whereas the positive control group (Control HC) had the hypercholestrol diet (basal diet plus 1% cholesterol and 0.5% cholic acid). Two experimental groups (2.5 RES and 5 RES) received resveratrol with doses of 2.5 and 5 mg/kg separately in addition to their basal diet. The other two test groups were given the HC diet while treated with 2.5 and 5 mg/kg of resveratrol (2.5 RES-HC and 5 RES-HC).
Chemical Analysis
In the end of the trial, rats were anaesthetized and blood samples were collected and centrifuged to prepare the serum plasma. Liver tissues were immediately extracted and kept in -80°C for future experiments. The plasma levels of total cholesterol (TC), triglyceride (TG), high density lipoprotein (HDL), malondialdehyde (MDA), and low density lipoprotein (LDL) were evaluated by available diagnostic kits (Pars Azmoon, Tehran, Iran). The levels of glutathione peroxidase (GPx), glutathione reductase (GR), paraoxonase-1 and sulfiredoxin-1 were assessed as it was previously reported by Al-Rejaie et al. [22]. The primers sequences were GPx, forward (5'- GGTGTTCCAGTGCGCAGAT-3') and reverse (5'-AGGGCTTCTATATCGGGTTCGA-3'); GR, forward (5'-TGAGCCGCCTGAACAACA-3') and reverse (5'-TTGCGTAGCCGTGGATGAC-3'); Paraoxonase-1, forward (5′- TGAGAGCTTCTATGCCACAAATG-3′) and reverse (5′-CCATGACAGGCCCAAGTACA-3′); Sulfiredoxin-1, forward (5′- AATCCCCAACCCCTGACTTT-3′) and reverse (5′- TGAACTGACCAGTGGAGACACAGT-3′).
Statistical Analysis
One way analysis of variance following by the Tukey-Kramer multiple comparison test was used to compare the data.
Findings
Biochemical parameters
The plasma concentrations of cholesterol, triglycerides, LDL-C and malondialdehyde were significantly higher and the level of HDL was significantly lower in hypercholesterolemic rats (p<0.05; Table 1).
Table 1. Effects of resveratrol on biochemical parameters in hypercholesterolemic rats


Figure 1. Effects of resveratrol on antioxidant status in hypercholesterolemic rats
Dietary supplementation with resveratrol, especially in higher level (5mg/kg) could decrease the levels of cholesterol, triglycerides, LDL-C and malondialdehyde and increase the HDL-C level (p<0.05). Dietary inclusion of resveratrol had no significant effects on blood parameters in healthy rats (p>0.05).
Antioxidant status
Effects of resveratrol on antioxidant status in hypercholesterolemic rats are depicted in figure 1. Hypercholesterolemic rats showed a decrease in GPx and GR and an increase in paraoxonase-1 and sulfiredoxin-1 in comparison to healthy control (p<0.05), but administration of resveratrol into diet could reverse these changes (p<0.05). There was no significant difference between treated and healthy rats in mentioned parameters (p>0.05).
Discussion
Obesity has been known as a risk factor for some diseases including cardiovascular and liver diseases [3, 4]. Hypercholesterolemic A suitable animal model for human obesity syndrome is a rat with hypercholesterolemia [23]. Our findings showed that lipid profile was changed in rats with hypercholestrolemia. Lipid profile changes have been known as a contributory factor in oxidative stress related to obesity that is caused by an increase in the production of ROS as well as a decrease in antioxidant enzymes [24]. ROSs and lipid peroxidation products disturb the respiratory chain in hepatocytes by oxidative damage to the mitochondrial DNA. Previous studies have reported a change in lipid profile of hypercholesterolemic rats [25]. High cholesterol diet may cause dyslipidemia syndrome and hyperlipidemia that is characterized by increased triglycerides in addition to decreased HDL-C [26]. Such results were also observed in the current study. Our findings showed that resveratrol could improve lipid profile. It has been previously reported that resveratrol was responsible for an improvement in serum lipids [27].
Resveratrol has also been reported to have antioxidant effects by reducing H2O2 and lipid peroxidation in the skin [28]. Previous studies have shown that treatment with resveratrol improves atherosclerosis through decreasing lipid drops in the intima of the aorta and also reducing vascular oxidative stress [29]. Our findings showed that hypercholesterolemia had elevated lipid peroxidation in hepatic tissue which is highlighted by increased plasma level of MDA. MDA increases the accumulation of H2O2 and finally promotes the lipid peroxidation. Our observations suggested that resveratrol reduces the level of MDA and improves the lipid profile that is probably as a result of decreased MDA.
Antioxidant system plays a significant role in the detoxification process in the liver. GPx is a selenoenzyme that catalyzes the reduction of hydrogen peroxide to H2O [30]. Down-regulation of GR provoke some responses that increases oxidative stress. Our findings showed that hypercholesterolemia increases the production of free radicals and also decreases the ability to detoxify ROS which leads to hepatocellular damage [31]. These findings show that resveratrol has antioxidant activity. Paraoxonase-1 is an enzyme with lactonase and esterase activities which is produced in the liver [32] and is known to have a role in regulating oxidative stress, fibrosis and hepatic cell apoptosis in chronic liver diseases [33]. Increased paraoxonase-1 expression develop the sensitivity to liver damage, chronic hepatitis and liver cirrhosis. Sulfiredoxin-1 is an antioxidant enzyme which contains a C-terminal cysteine residue and is essential for its antioxidant activity [34]. It is also known to play a role in cellular responses to oxidative stress through restoring the activity of over-oxidized peroxiredoxins [34]. These findings show importance of resveratrol for treating hepatitis and cirrhosis and reducing the oxidative stress.
Conclusion
Hypercholesterolemia causes imbalances in the lipid profile and antioxidant system but resveratrol in high doses reverses these adverse effects.
Acknowledgements: None declared by the authors.
Ethical Permission: Compliance with ethical guidelines Approval for this study was obtained from International Center for Intelligent Research Ethics Committee (Tbilisi, Georgia).
Conflicts of Interests: None declared by the authors.
Authors' Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: This study was supported by a grant from International Center for Intelligent Research (ICIR-2017-154789).
References
1. Otunola GA, Oloyede OB, Oladiji AT, Afolayan AA. Effects of diet-induced hypercholesterolemia on the lipid profile and some enzyme activities in female Wistar rats. Afr J Biochem Res. 2010;4(6):149-54. [Link]
2. Matos SL, Paula H, Pedrosa ML, Santos RC, Oliveira EL, Chianca Jr DA, et al. Dietary models for inducing hypercholesterolemia in rats. Braz Arch Biol Technol. 2005;48(2):203-9. [Link] [DOI:10.1590/S1516-89132005000200006]
3. Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132(2):112-7. [Link] [DOI:10.7326/0003-4819-132-2-200001180-00004]
4. Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A, et al. Coffee reduces liver damage in a rat model of steatohepatitis: The underlying mechanisms and the role of polyphenols and melanoidins. Hepatology. 2010;52(5):1652-61. [Link] [DOI:10.1002/hep.23902]
5. Araya J, Rodrigo R, Pettinelli P, Araya AV, Poniachik J, Videla LA. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity (Silver Spring). 2010;18(7):1460-3. [Link] [DOI:10.1038/oby.2009.379]
6. Videla LA, Rodrigo R, Araya J, Poniachik J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol Med. 2006;12(12):555-8. [Link] [DOI:10.1016/j.molmed.2006.10.001]
7. Sonta T, Inoguchi T, Tsubouchi H, Sekiguchi N, Kobayashi K, Matsumoto S, et al. Evidence for contribution of vascular NAD(P)H oxidase to increased oxidative stress in animal models of diabetes and obesity. Free Radic Biol Med. 2004;37(1):115-23. [Link] [DOI:10.1016/j.freeradbiomed.2004.04.001]
8. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114(12):1752-61. [Link] [DOI:10.1172/JCI21625]
9. Faienza MF, Francavilla R, Goffredo R, Ventura A, Marzano F, Panzarino G, et al. Oxidative stress in obesity and metabolic syndrome in children and adolescents. Horm Res Paediatr. 2012;78(3):158-64. [Link] [DOI:10.1159/000342642]
10. Park S, Kim M, Paik JK, Jang YJ, Lee SH, Lee JH. Oxidative stress is associated with C-reactive protein in non-diabetic postmenopausal women, independent of obesity and insulin resistance. Clin Endocrinol. 2012;79(1):65-70. [Link] [DOI:10.1111/j.1365-2265.2012.04512.x]
11. Mishra KP. Cell membrane oxidative damage induced by gammaradiation and apoptotic sensitivity. J Environ Pathol Toxicol Oncol. 2004;23(1):61-6. [Link] [DOI:10.1615/JEnvPathToxOncol.v23.i1.60]
12. Bravo E, Palleschi S, Aspichueta P, Buque X, Rossi B, Cano A, et al. High fat diet-induced non alcoholic fatty liver disease in rats is associated with hyperhomocysteinemia caused by down regulation of the transsulphuration pathway. Lipids Health Dis. 2011;10:60. [Link] [DOI:10.1186/1476-511X-10-60]
13. Koc A, Duru M, Ciralik H, Akcan R, Sogut S. Protective agent, erdosteine, against cisplatin-induced hepatic oxidant injury in rats. Mol Cell Biochem. 2005;278(1-2):79-84. [Link] [DOI:10.1007/s11010-005-6630-z]
14. Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot. 2003;91 Spec No(2):179-94. [Link] [DOI:10.1093/aob/mcf118]
15. Yagmurca M, Bas O, Mollaoglu H, Sahin O, Nacar A, Karaman O, et al. Protective effects of erdosteine on doxorubicin-induced hepatotoxicity in rats. Arch Med Res. 2007;38(4):380-5. [Link] [DOI:10.1016/j.arcmed.2007.01.007]
16. Sayed Ahmed MM, Aleisa AM, Al Rejaie SS, Al Yahya AA, Al Shabanah OA, Hafez MM, et al. Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signaling. Oxid Med Cell Longev. 2010;3(4):254-61. [Link] [DOI:10.4161/oxim.3.4.12714]
17. Senti M, Tomas M, Fito M, Weinbrenner T, Covas MI, Sala J, et al. Antioxidant paraoxonase 1 activity in the metabolic syndrome. J Clin Endocrinol Metab. 2003;88(11):5422-6. [Link] [DOI:10.1210/jc.2003-030648]
18. Peltoniemi MJ, Rytila PH, Harju TH, Soini YM, Salmenkivi KM, Ruddock LW, et al. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res. 2006;7(1):133. [Link] [DOI:10.1186/1465-9921-7-133]
19. Lu W, Chen Z, Zhang H, Wang Y, Luo Y, Huang P. ZNF143 transcription factor mediates cell survival through upregulation of the GPX1 activity in the mitochondrial respiratory dysfunction. Cell Death Dis. 2012;3:e422. [Link] [DOI:10.1038/cddis.2012.156]
20. Sharma R, Sharma NK, Thungapathra M. Resveratrol regulates body weight in healthy and ovariectomized rats. Nutr Metab (Lond). 2017;14:30. [Link] [DOI:10.1186/s12986-017-0183-5]
21. Rivera L, Morón R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77(6):1053-63. [Link] [DOI:10.1016/j.bcp.2008.11.027]
22. Al-Rejaie SS, Aleisa AM, Sayed-Ahmed MM, AL-Shabanah OA, Abuohashish HM, Ahmed MM, et al. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complement Altern Med. 2013;13:136. [Link] [DOI:10.1186/1472-6882-13-136]
23. Madsen AN, Hansen G, Paulsen SJ, Lykkegaard K, Tang Christensen M, Hansen HS, et al. Long-term characterization of the diet-induced obese and diet-resistant rat model: A polygenetic rat model mimicking the human obesity syndrome. J Endocrinol. 2010;206(3):287-96. [Link] [DOI:10.1677/JOE-10-0004]
24. Tsimikas S, Miller YI. Oxidative modification of lipoproteins: Mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr Pharm Des. 2011;17(1):27-37. [Link] [DOI:10.2174/138161211795049831]
25. Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr. 2011;3(1):17. [Link] [DOI:10.1186/1758-5996-3-17]
26. Bloomgarden ZT. Dyslipidemia and the metabolic syndrome. Diabetes Care 2004;27(12):3009-16. [Link] [DOI:10.2337/diacare.27.12.3009]
27. Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49-52. [Link] [DOI:10.5344/ajev.1992.43.1.49]
28. Afaq F, Adhami VM, Ahmad N. Prevention of short-term ultraviolet B radiationmediated damages by resveratrol in SKH-1 hairless mice. Toxicol Appl Pharmacol.2003;186(1):28-37. [Link] [DOI:10.1016/S0041-008X(02)00014-5]
29. Azorín Ortuño M, Yañéz Gascón MJ, Pallarés FG, Rivera J, González Sarrías A, Larrosa M, et al. A dietary resveratrol-rich grape extract prevents the developing of atherosclerotic lesions in the aorta of pigs fed an atherogenic diet. J Agric Food Chem. 2012;60(22):5609-20. [Link] [DOI:10.1021/jf301154q]
30. Tappel AL. Glutathione peroxidase and hydroperoxides. Methods Enzymol. 1978;52:506-13. [Link] [DOI:10.1016/S0076-6879(78)52055-7]
31. Pradeep K, Mohan CV, Gobianand K, Karthikeyan S. Silymarin modulates the oxidant-antioxidant imbalance during diethylnitrosamine induced oxidative stress in rats. Eur J Pharmacol. 2007;560(2-3):110-6. [Link] [DOI:10.1016/j.ejphar.2006.12.023]
32. Rodrigo L, Gil F, Hernandez AF, Marina A, Vazquez J, Pla A. Purification and characterization of paraoxon hydrolase from rat liver. Biochem J. 1997;321(Pt 3):595-601. [Link] [DOI:10.1042/bj3210595]
33. Ferre N, Marsillach J, Camps J, Mackness B, Mackness M, Riu F, et al. Paraoxonase-1 is associated with oxidative stress, fibrosis and FAS expression in chronic liver diseases. J Hepatol. 2006;45(1):51-9. [Link] [DOI:10.1016/j.jhep.2005.12.018]
34. Rhee SG, Jeong W, Chang TS, Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: Its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007;(106):S3-8. [Link] [DOI:10.1038/sj.ki.5002380]









