GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 2 (2024)
GMJM 2024, 3(2): 63-67 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2023/11/2 | Accepted: 2024/03/1 | Published: 2024/04/24
Received: 2023/11/2 | Accepted: 2024/03/1 | Published: 2024/04/24
How to cite this article
Jazideh F, Tarkhnishvili E, Hashemi Feyzabadi S. Effects of Olea europaea L. Extract on Inflammatory Gene Expressions in Infected Wound Healing Process in Mice Model. GMJM 2024; 3 (2) :63-67
URL: http://gmedicine.de/article-2-226-en.html
URL: http://gmedicine.de/article-2-226-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Department of Medical Research, Institute for Intelligent Research, Tbilisi, Georgia
2- Semnan University of Medical Sciences, Semnan, Iran
2- Semnan University of Medical Sciences, Semnan, Iran
Keywords:
| Abstract (HTML) (1281 Views)
Full-Text: (421 Views)
Introduction
One of the challenges that people commonly face is wounds [1]. It is estimated that 6 million people suffer from chronic wounds all over the world [2]. A wound is defined as a damaged condition of tissue that is created through chemical, physical, microbial, or immunological disorders or typically related to loss of function [3]. Wounds are also defined as physical damages that cause an opening or break of the skin, disturbing the normal skin performance and anatomy [4]. Bacterial infection delays wound healing process [5]. The most common and unavoidable obstruction to wound healing is the induction of infection, especially in chronic wounds [6]. Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), and Pseudomonas aeruginosa are known as microbial strains that are commonly found in individuals with infected wounds. The wound healing process comprises several continuous and sequential steps, including hemostasis, inflammation, proliferation, and remodeling. Faults in these steps impair wound healing and increase healthiness and economic problems [7, 8]. Inflammation occurs following the hemostasis step. Hemostasis lasts for several hours following injury, and inflammation lasts from 1 up to 3 days after injury [9]. In the inflammation step, following activation of the immune system, neutrophils and monocytes immediately move into the damaged skin. The inflammation occurs concurrent with the hemostasis phase, and both are early steps of wound healing [10]. Excessive and prolonged inflammation delays wound healing and increases the production of the wound [9]. Shortening the inflammatory phase can accelerate the wound-healing process [12].
The use of topical antimicrobial agents such as silver sulfadiazine can decrease the risk of infection during wound healing [11, 12]. Olive (Olea europaea L.) has active compounds, including oleuropein, rutin, luteolin, apigenin, triterpenes, and chalcones [13].
It is known to have antioxidant and anti-inflammatory properties [14]. Seemingly, olive can improve wound healing due to its anti-inflammatory properties. This study was conducted for the first time to evaluate the effects of ointments prepared from olive leaf extract on inflammatory gene expressions in the infected wound healing process in mice models.
Materials and Methods
Preparation of olive extract
Olea europaea L. leaves were prepared from southern regions of Iran, washed, and air-dried in the shade. We ground dry leaves were ground into a fine powder and soaked in 80% ethanol for 24h. After filtration, the solvent was evaporated at a temperature not exceeding 45°C by a rotary evaporator, as suggested by previous studies [15].
Induction of wound and the treatment
In the current study, a total number of 60 BALB/c mice with an initial weight of 30±5g were purchased from Pasture Institute and grouped into four groups. The mice were divided into four groups. A group was selected as a negative control that was considered an infected group without any treatment. Three other groups were considered infected and treated with mupirocin ointment as the control standard, and ointments were prepared from olive extract. To induce wound, animals were anesthetized, the dorsum back hairs were shaved, and the skin scrubbed. We created two circular full-thickness wounds on the dorsal interscapular part of the mouse with a 5mm biopsy punch. Following the creation of the wound, 50µl of suspension containing 25×107 cells of Gram-positive species S. aureus (strain ATCC 25923) and Gram-negative species P. aeruginosa (strain ATCC 27853) were inoculated per wound [12]. Animals were treated with mupirocin (mupirocin group) and basal ointments containing 2.5% and 5% of the extract (2.5% & 5% OLE). The animals were treated with ointments 24 hours after creating wounds. In the current study, five mice per group were selected, and samples were collected on days 3, 7, and 14 for investigation of total bacterial count and gene expressions. We measured the wound area with a graph sheet as described by Farahpour et al. [12].
Investigation of total bacterial count
We collected the samples, and 0.1g of the sample was crushed and homogenized in a sterile mortar containing 10ml of sterile saline, serially diluted in the tube containing 9ml of sterile saline. The samples were cultured on plate count agar (Merck KGaA, Darmstadt, Germany) and superficially duplicated. Following incubation, all colonies were counted and reported as CFU/g of granulation tissue [12].
RNA extraction and quantitative real-time PCR
After the wound's creation, samples were collected for evaluating the gene expression profile on days 3, 7, and 14. In the current study, 3-5g of wound tissues were transferred into tubes containing RNase solution (Qiagen; Germany) and rapidly transferred to the lab. Following homogenization, RNA was extracted using the Trizol method (Roche; Germany). We synthesized cDNA using the Exiqon cDNA Synthesis Kit based on the manufacturer's instructions. Samples were rapidly incubated at 25°C for 5min, then at 42°C for 60min; the reaction was finally terminated by heating at 70°C for 5min. Light Cycler 96 Roche was used to assess the mRNA expression, and primers were used for IL-10, IL-1β, TNF-α, and TGF-β1 genes. The primers sequences were as follows: IL-10, forward (5′-CCA TCA TGC CTG GCT CAG CAC-3′) and reverse (5′-TGT ACT GGC CCC TGC TGA TCC-3′); IL-1β, forward (5′-AAC AAA CCC TGC AGT GGT TCG-3′) and reverse (5′- AGC TGC TTC AGA CAC TTG CAC-3′); TNF-α, forward (5′-GAA GCT CCC TCA GCG AGG ACA-3′) and reverse (5′-TTG GGC CAG TGA GTG AAA GGG-3′); TGF-β1, forward (5′-CTG AAC CAA GGA GAC GGA AT-3′) and reverse (5′-GGT TCA TGT CAT GGA TGG TG-3′).
Data analysis
The data were analyzed using SPSS 21 software and reported as the mean ± standard deviation. The ANOVA procedure was used to analyze the data, and the Tukey test was used to compare the data. Changes in the fold number were evaluated using the 2-ΔΔCt method.
Findings
Wound area
The wound area was not influenced by experimental treatments on day 3 (p>0.05). The wound area was significantly higher in the control group compared with other groups on days 7 and 14 (p<0.05). The treatment with mupirocin and OLE in both levels significantly decreased wound area, and the best responses were observed in 5% OLE treatment (p<0.05; Figure 1).
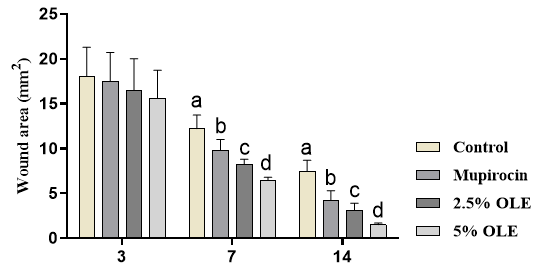
Figure 1. The effects of experimental treatments on wound area. The results showed that OLE and mupirocin significantly decreased wound area. Superscripts (a–d) show significant differences among groups at p<0.05.
Total bacterial count
The total bacterial count was not affected by the treatments at day 3 (p>0.05). The results showed that total bacterial count was significantly higher in control mice compared to other mice at days 7 and 14 (p<0.05). The results show that the treatment with OLE and mupirocin significantly decreased total bacterial count, and the best responses were observed in the treatment of 5% OLE (Figure 2).
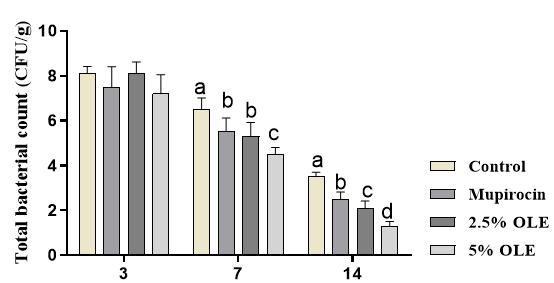
Figure 2. The effects of experimental treatments on total bacterial count. The results showed that OLE and mupirocin significantly decreased total bacterial count. Superscripts (a–d) show significant differences among groups at P<0.05.
Inflammatory gene expressions
The results for inflammatory genes are shown in Figure 3. The results showed that inflammatory genes are shown in Figure 3. The results showed that experimental treatments did not influence gene expressions in day 3 (p>0.05). The results showed that OLE and mupirocin significantly decreased the expression of IL-1β and TNF-α and increased the expression of TGF-β and IL-10 compared to the control group at days 7 and 14 (p<0.05). The results showed higher expression for TGF-β and IL-10 were observed in days 7 and 14 (p<0.05).
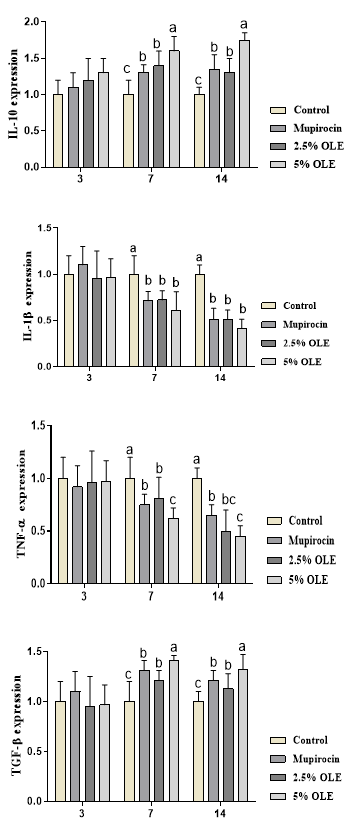
Figure 3. The effects of experimental treatments on total inflammatory genes expression. The results showed that OLE and mupirocin significantly decreased the expression of IL-1β and TNF-α and increased the expression of TGF-β and IL-10. Superscripts (a–c) show significant differences among groups at P<0.05.
Discussion
This study was conducted to evaluate the effects of ointments prepared from OLE. The results showed that ointments prepared from OLE decreased inflammatory phase, total bacterial count, and wound healing. It is well accepted that inflammatory reaction cascades play important roles in wound healing process. Inflammation is commonly initiated by harm to living tissues due to injury caused by infections induced by live organisms such as bacteria and/or physical agents; and faulted immune response. The basal purpose of inflammatory response is to remove the foreign agents and injured tissue parts for improving healing of the influenced tissues, organs, or system [16, 17]. Activated host immune system increases fight infection, whereas overproduced inflammation causes to produce the tissue damage or even multiple organ failure. Overproduction of pro-inflammatory cytokines delays wound healing process. It is known that innate immune system is involved in the defense system through activating the infiltration of the immune cells, including neutrophils, macrophages, and dendritic cells for phagocytosis of infectious pathogens to the site of injury when the elementary stages of the process of wound healing [18]. The results showed that OLE could decrease total bacterial count. Decreasing microbial load of the wound and lowering the tissue inflammation induce the second stage of wound healing [19]. Decreased total bacterial count could be attributed to antibacterial properties of OLE that significantly decrease total bacterial count. The results show that OLE significantly decreases total bacterial count and promotes wound healing process and proliferative phase. Bacteria are found in all the open dermal wounds and a contact between bacteria and some hosts from contamination by colonization on local infection increases infection by cellulitis and/or septicemia [20]. Bacterial infections usually complicate wound healing process that keeps successive influx of neutrophils and macrophages and delays wound healing process [20]. Decreased inflammatory phase cause the migration of fibroblasts to the wound site, collagen production, and increase the epithelial cells from the edge to the wound site [12]. The results show that OLE improves wound healing by modulation in inflammatory phase genes. In following, we will discuss the genes.
TNF-𝛼 is a pleiotropic cytokine that is produced by a group of cells such as keratinocytes, macrophages, and mast cells. It is involved in some mechanisms such as leukocyte recruitment mainly neutrophils, inducing control of molecular adhesion, production of chemokines, and metalloproteinases matrix and also tissue preventors of metalloproteinases. The TNF-α is known inflammatory cytokine and have important roles in the first stage the inflammation [12]. The IL-1β is known as an inflammatory molecule that induces the aggregate of neutrophils to the site of infection [21]. The TNF- and IL-1β prolongs inflammatory stage inflammatory phase during wound healing and delay wound healing [22]. TGF-β1 activates the fibroblasts and begins the proliferative phase [23]. It was reported the role of TGF-β1 for participating in angiogenesis, regulating the granulation tissue formation [24] and re-epithelialization [25]. In addition, increase in the expression of IL-10 increases activity of macrophages in the wound site and promotes wound healing [26, 27]. In addition, increased IL-10 enhances activity of macrophages in the wound site and improves wound healing [26, 27].
Conclusion
In sum, ointments prepared from OLE decreased inflammatory phase and bacterial count and hastens wound healing. We suggest to use it for the treatment of infected wounds in combination with synthetic agents.
Acknowledgements: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
One of the challenges that people commonly face is wounds [1]. It is estimated that 6 million people suffer from chronic wounds all over the world [2]. A wound is defined as a damaged condition of tissue that is created through chemical, physical, microbial, or immunological disorders or typically related to loss of function [3]. Wounds are also defined as physical damages that cause an opening or break of the skin, disturbing the normal skin performance and anatomy [4]. Bacterial infection delays wound healing process [5]. The most common and unavoidable obstruction to wound healing is the induction of infection, especially in chronic wounds [6]. Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), and Pseudomonas aeruginosa are known as microbial strains that are commonly found in individuals with infected wounds. The wound healing process comprises several continuous and sequential steps, including hemostasis, inflammation, proliferation, and remodeling. Faults in these steps impair wound healing and increase healthiness and economic problems [7, 8]. Inflammation occurs following the hemostasis step. Hemostasis lasts for several hours following injury, and inflammation lasts from 1 up to 3 days after injury [9]. In the inflammation step, following activation of the immune system, neutrophils and monocytes immediately move into the damaged skin. The inflammation occurs concurrent with the hemostasis phase, and both are early steps of wound healing [10]. Excessive and prolonged inflammation delays wound healing and increases the production of the wound [9]. Shortening the inflammatory phase can accelerate the wound-healing process [12].
The use of topical antimicrobial agents such as silver sulfadiazine can decrease the risk of infection during wound healing [11, 12]. Olive (Olea europaea L.) has active compounds, including oleuropein, rutin, luteolin, apigenin, triterpenes, and chalcones [13].
It is known to have antioxidant and anti-inflammatory properties [14]. Seemingly, olive can improve wound healing due to its anti-inflammatory properties. This study was conducted for the first time to evaluate the effects of ointments prepared from olive leaf extract on inflammatory gene expressions in the infected wound healing process in mice models.
Materials and Methods
Preparation of olive extract
Olea europaea L. leaves were prepared from southern regions of Iran, washed, and air-dried in the shade. We ground dry leaves were ground into a fine powder and soaked in 80% ethanol for 24h. After filtration, the solvent was evaporated at a temperature not exceeding 45°C by a rotary evaporator, as suggested by previous studies [15].
Induction of wound and the treatment
In the current study, a total number of 60 BALB/c mice with an initial weight of 30±5g were purchased from Pasture Institute and grouped into four groups. The mice were divided into four groups. A group was selected as a negative control that was considered an infected group without any treatment. Three other groups were considered infected and treated with mupirocin ointment as the control standard, and ointments were prepared from olive extract. To induce wound, animals were anesthetized, the dorsum back hairs were shaved, and the skin scrubbed. We created two circular full-thickness wounds on the dorsal interscapular part of the mouse with a 5mm biopsy punch. Following the creation of the wound, 50µl of suspension containing 25×107 cells of Gram-positive species S. aureus (strain ATCC 25923) and Gram-negative species P. aeruginosa (strain ATCC 27853) were inoculated per wound [12]. Animals were treated with mupirocin (mupirocin group) and basal ointments containing 2.5% and 5% of the extract (2.5% & 5% OLE). The animals were treated with ointments 24 hours after creating wounds. In the current study, five mice per group were selected, and samples were collected on days 3, 7, and 14 for investigation of total bacterial count and gene expressions. We measured the wound area with a graph sheet as described by Farahpour et al. [12].
Investigation of total bacterial count
We collected the samples, and 0.1g of the sample was crushed and homogenized in a sterile mortar containing 10ml of sterile saline, serially diluted in the tube containing 9ml of sterile saline. The samples were cultured on plate count agar (Merck KGaA, Darmstadt, Germany) and superficially duplicated. Following incubation, all colonies were counted and reported as CFU/g of granulation tissue [12].
RNA extraction and quantitative real-time PCR
After the wound's creation, samples were collected for evaluating the gene expression profile on days 3, 7, and 14. In the current study, 3-5g of wound tissues were transferred into tubes containing RNase solution (Qiagen; Germany) and rapidly transferred to the lab. Following homogenization, RNA was extracted using the Trizol method (Roche; Germany). We synthesized cDNA using the Exiqon cDNA Synthesis Kit based on the manufacturer's instructions. Samples were rapidly incubated at 25°C for 5min, then at 42°C for 60min; the reaction was finally terminated by heating at 70°C for 5min. Light Cycler 96 Roche was used to assess the mRNA expression, and primers were used for IL-10, IL-1β, TNF-α, and TGF-β1 genes. The primers sequences were as follows: IL-10, forward (5′-CCA TCA TGC CTG GCT CAG CAC-3′) and reverse (5′-TGT ACT GGC CCC TGC TGA TCC-3′); IL-1β, forward (5′-AAC AAA CCC TGC AGT GGT TCG-3′) and reverse (5′- AGC TGC TTC AGA CAC TTG CAC-3′); TNF-α, forward (5′-GAA GCT CCC TCA GCG AGG ACA-3′) and reverse (5′-TTG GGC CAG TGA GTG AAA GGG-3′); TGF-β1, forward (5′-CTG AAC CAA GGA GAC GGA AT-3′) and reverse (5′-GGT TCA TGT CAT GGA TGG TG-3′).
Data analysis
The data were analyzed using SPSS 21 software and reported as the mean ± standard deviation. The ANOVA procedure was used to analyze the data, and the Tukey test was used to compare the data. Changes in the fold number were evaluated using the 2-ΔΔCt method.
Findings
Wound area
The wound area was not influenced by experimental treatments on day 3 (p>0.05). The wound area was significantly higher in the control group compared with other groups on days 7 and 14 (p<0.05). The treatment with mupirocin and OLE in both levels significantly decreased wound area, and the best responses were observed in 5% OLE treatment (p<0.05; Figure 1).

Figure 1. The effects of experimental treatments on wound area. The results showed that OLE and mupirocin significantly decreased wound area. Superscripts (a–d) show significant differences among groups at p<0.05.
Total bacterial count
The total bacterial count was not affected by the treatments at day 3 (p>0.05). The results showed that total bacterial count was significantly higher in control mice compared to other mice at days 7 and 14 (p<0.05). The results show that the treatment with OLE and mupirocin significantly decreased total bacterial count, and the best responses were observed in the treatment of 5% OLE (Figure 2).

Figure 2. The effects of experimental treatments on total bacterial count. The results showed that OLE and mupirocin significantly decreased total bacterial count. Superscripts (a–d) show significant differences among groups at P<0.05.
Inflammatory gene expressions
The results for inflammatory genes are shown in Figure 3. The results showed that inflammatory genes are shown in Figure 3. The results showed that experimental treatments did not influence gene expressions in day 3 (p>0.05). The results showed that OLE and mupirocin significantly decreased the expression of IL-1β and TNF-α and increased the expression of TGF-β and IL-10 compared to the control group at days 7 and 14 (p<0.05). The results showed higher expression for TGF-β and IL-10 were observed in days 7 and 14 (p<0.05).

Figure 3. The effects of experimental treatments on total inflammatory genes expression. The results showed that OLE and mupirocin significantly decreased the expression of IL-1β and TNF-α and increased the expression of TGF-β and IL-10. Superscripts (a–c) show significant differences among groups at P<0.05.
Discussion
This study was conducted to evaluate the effects of ointments prepared from OLE. The results showed that ointments prepared from OLE decreased inflammatory phase, total bacterial count, and wound healing. It is well accepted that inflammatory reaction cascades play important roles in wound healing process. Inflammation is commonly initiated by harm to living tissues due to injury caused by infections induced by live organisms such as bacteria and/or physical agents; and faulted immune response. The basal purpose of inflammatory response is to remove the foreign agents and injured tissue parts for improving healing of the influenced tissues, organs, or system [16, 17]. Activated host immune system increases fight infection, whereas overproduced inflammation causes to produce the tissue damage or even multiple organ failure. Overproduction of pro-inflammatory cytokines delays wound healing process. It is known that innate immune system is involved in the defense system through activating the infiltration of the immune cells, including neutrophils, macrophages, and dendritic cells for phagocytosis of infectious pathogens to the site of injury when the elementary stages of the process of wound healing [18]. The results showed that OLE could decrease total bacterial count. Decreasing microbial load of the wound and lowering the tissue inflammation induce the second stage of wound healing [19]. Decreased total bacterial count could be attributed to antibacterial properties of OLE that significantly decrease total bacterial count. The results show that OLE significantly decreases total bacterial count and promotes wound healing process and proliferative phase. Bacteria are found in all the open dermal wounds and a contact between bacteria and some hosts from contamination by colonization on local infection increases infection by cellulitis and/or septicemia [20]. Bacterial infections usually complicate wound healing process that keeps successive influx of neutrophils and macrophages and delays wound healing process [20]. Decreased inflammatory phase cause the migration of fibroblasts to the wound site, collagen production, and increase the epithelial cells from the edge to the wound site [12]. The results show that OLE improves wound healing by modulation in inflammatory phase genes. In following, we will discuss the genes.
TNF-𝛼 is a pleiotropic cytokine that is produced by a group of cells such as keratinocytes, macrophages, and mast cells. It is involved in some mechanisms such as leukocyte recruitment mainly neutrophils, inducing control of molecular adhesion, production of chemokines, and metalloproteinases matrix and also tissue preventors of metalloproteinases. The TNF-α is known inflammatory cytokine and have important roles in the first stage the inflammation [12]. The IL-1β is known as an inflammatory molecule that induces the aggregate of neutrophils to the site of infection [21]. The TNF- and IL-1β prolongs inflammatory stage inflammatory phase during wound healing and delay wound healing [22]. TGF-β1 activates the fibroblasts and begins the proliferative phase [23]. It was reported the role of TGF-β1 for participating in angiogenesis, regulating the granulation tissue formation [24] and re-epithelialization [25]. In addition, increase in the expression of IL-10 increases activity of macrophages in the wound site and promotes wound healing [26, 27]. In addition, increased IL-10 enhances activity of macrophages in the wound site and improves wound healing [26, 27].
Conclusion
In sum, ointments prepared from OLE decreased inflammatory phase and bacterial count and hastens wound healing. We suggest to use it for the treatment of infected wounds in combination with synthetic agents.
Acknowledgements: None declared by the authors.
Ethical Permission: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Funding/Support: None declared by the authors.
References
1. Nagori BP, Solanki R. Role of medicinal plants in wound healing. Res J Med Plant. 2011;5(4):392-405. [Link] [DOI:10.3923/rjmp.2011.392.405]
2. Kumar B, Govindarajan M, Pusphagandan R. Ethanopharmacological approaches to wound healing-exploring medicinal plants of India. J Ethanopharmacol. 2007;114(2):103-13. [Link] [DOI:10.1016/j.jep.2007.08.010]
3. Adhav R, Mantry P, Darwhekar GN. Wound healing medicinal plant of India: A review. Int J Pharmacogn. 2015;2(1):6-10. [Link]
4. Strodtbeck F. Physiology of wound healing. Newborn Infant Nurs Rev. 2001;1(1):43-5. [Link] [DOI:10.1053/nbin.2001.23176]
5. Khezri K, Farahpour MR, Mounesi Rad S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif Cells Nanomed Biotechnol. 2019;47:980-8. [Link] [DOI:10.1080/21691401.2019.1582539]
6. Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23:2392. [Link] [DOI:10.3390/molecules23092392]
7. Mustoe TA, O'Shaughnessy K, Kloeters O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast Reconstr Surg. 2006;117(7 Suppl):35S-41S. [Link] [DOI:10.1097/01.prs.0000225431.63010.1b]
8. Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763-71. [Link] [DOI:10.1111/j.1524-475X.2009.00543.x]
9. Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: A critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861-85. [Link] [DOI:10.1007/s00018-016-2268-0]
10. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. [Link] [DOI:10.1126/scitranslmed.3009337]
11. Modarresi M, Farahpour MR, Baradaran B. Topical application of Mentha piperita essential oil accelerates wound healing in infected mice model. Inflammopharmacology. 2019;27:531-7. [Link] [DOI:10.1007/s10787-018-0510-0]
12. Farahpour MR, Vahid M, Oryan A. Effectiveness of topical application of ostrich oil on the healing of Staphylococcus aureus- and Pseudomonas aeruginosa-infected wounds. Connect. Tissue Res. 2018;59(3):212-22. [Link]
13. Meirinhos J, Silva BM, Valentão P, Seabra RM, Pereira JA, Dias A, et al. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea Europaea L.) leaf cultivars. Nat Prod Res. 2005;19(2):189-95. [Link] [DOI:10.1080/14786410410001704886]
14. Pereira AP, Ferreira IC, Marcelino F, Valentao P, Andrade PB, Seabra R, et al. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrancosa) leaves. Molecules. 2007;12(5):1153-62. [Link] [DOI:10.3390/12051153]
15. Elgebaly HA, Mosa NM, Allach M, El-massry KF, El-Ghorab AE, Al Hroob AM, et al. Olive oil and leaf extract prevent fluoxetine-induced hepatotoxicity by attenuating oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018;98:446-53. [Link] [DOI:10.1016/j.biopha.2017.12.101]
16. Ahmed AU. An overview of inflammation: Mechanism and consequences. Front Biol. 2011;6(4):274-81. [Link] [DOI:10.1007/s11515-011-1123-9]
17. Garrett WS, Gordon JI, Glimber LH. Homeostasis and inflammation in the intestine. Cell. 2010;140(6):859-70. [Link] [DOI:10.1016/j.cell.2010.01.023]
18. Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6(3):173-82. [Link] [DOI:10.1038/nri1785]
19. Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: Recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;6:1-23. [Link] [DOI:10.1007/s00018-012-1152-9]
20. Edwards R, Harding KG. Bacteria and wound healing. Curr Opin Infect Dis. 2004;17(2):91-6. [Link] [DOI:10.1097/00001432-200404000-00004]
21. Eo H, Lee H-J, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478:1021-7. [Link] [DOI:10.1016/j.bbrc.2016.07.039]
22. Hozzeina WN, Badrc G, Al Ghamdid A, Sayede A, Al-Wailif NS, Garraudg O. Topical application of propolis enhances cutaneous wound healing by promoting TGF-Beta/smad-mediated collagen production in a streptozotocin-induced type I diabetic mouse model. Cell Physiol Biochem. 2015;37:940-54. [Link] [DOI:10.1159/000430221]
23. Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPINas a unifyin gnomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468-71. [Link] [DOI:10.1194/jlr.R000034]
24. Okuda K, Murata M, Sugimoto M, Saito Y, Kabasawa Y, Yoshie H, et al. TGF-beta1 influencese arlygingi val wound healing in rats: Animmuno- histochemical evaluation of stromal remodelling by extracellular matrix molecules and PCNA. J Oral Pathol Med. 1998;27:463-9. [Link] [DOI:10.1111/j.1600-0714.1998.tb01913.x]
25. Schmid P, Cox D, Bilbe G, McMaster G, Morrison C, Stähelin H, et al. TGF-betas and TGF-beta type II receptor in human epidermis: differential expression in acute and chronic skin wounds. J Pathol. 1993;171:191-7. [Link] [DOI:10.1002/path.1711710307]
26. Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun. 1999;265(1):194-9. [Link] [DOI:10.1006/bbrc.1999.1455]
27. Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukocyte Biol. 2001;69(4):513-21. [Link] [DOI:10.1189/jlb.69.4.513]









