GMJ Medicine
eISSN : 2626-3041
Volume 3, Issue 3 (2024)
GMJM 2024, 3(3): 99-102 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/01/16 | Accepted: 2024/06/5 | Published: 2024/07/11
Received: 2024/01/16 | Accepted: 2024/06/5 | Published: 2024/07/11
How to cite this article
Günther J, Schneider I, Krämer A. Carvone Prevents and Alleviates Hepatic Steatosis in Rat Model with Nonalcoholic Fatty Liver Disease. GMJM 2024; 3 (3) :99-102
URL: http://gmedicine.de/article-2-228-en.html
URL: http://gmedicine.de/article-2-228-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Carvone Prevents and Alleviates Hepatic Steatosis in Rat Model with Nonalcoholic Fatty Liver Disease
1- Department of Phytochemistry, Institute for Phytochemical Research (IPR), Berlin, Germany
Keywords:
| Abstract (HTML) (1153 Views)
Full-Text: (315 Views)
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a condition in which fat significantly aggregates in the liver of a patient who lacks a history of alcohol abuse [1].
NAFLD is grouped into two groups, including simple steatosis and nonalcoholic steatohepatitis (NASH). Under NASH conditions, steatosis, intralobular inflammation, and hepatocellular are found in progressive fibrosis [2]. Permanent NASH may be caused by liver cirrhosis and hepatocellular carcinoma [3-5]. NAFLD not only increases the risk of developing liver disease but is also one key component for metabolic syndrome, obesity, and type 2 diabetes [6]. NAFLD encompasses a broad range, including simple fatty liver (intracellular lipids >5%) up to progressive NASH, which is accompanied by lobular inflammation, fibrosis, and cirrhosis and increases the risk for hepatocellular carcinoma [7]. Increased triglyceride aggregation in hepatocytes indicates NAFLD, which is severely related to hepatic insulin resistance [8]. Increased triglyceride formation is observed in fatty livers accompanied by obesity and type 2 diabetes mellitus [9]. Hepatic fat aggregation causes hepatic insulin resistance by promoting gluconeogenesis and activating the PKC-ε and JNK1 signaling pathways [10]. It is not approved agents available for the treatment of NAFLD. Improvement of some factors, such as weight reduction and dietary fat intake, are usually known as treatment modalities in NAFLD disease [11]. Studies showed that insulin sensitizers, such as thiazolidinediones, and some antioxidants could improve clinical conditions of NASH [12, 13]. Carvone (5-isopropenyl-2-methyl-2-cyclohexenone) is one monocyclic monoterpene ketone that is found in 70 different plants. It is one of the main components of caraway oil. It is known to have biological activities, including antimicrobial [14], nematicidal [15], antitumor [16], and antioxidant [17] properties. It seems that carvone could improve and prevent hepatic steatosis in rat models of NAFLD due to its antioxidant properties. This study was thus conducted to evaluate the effects of carvone on hepatic steatosis and NAFLD by preventing hepatic triglyceride formation and oxidative processes.
Materials and Methods
Materials
Carvone was purchased from Sigma Chemicals Company, St. Louis, MO, USA, and kept at 2-4°C and protected from sunlight. Other chemicals were purchased from commercial suppliers.
Animal studies
A total number of 60 Wistar rats (180±10g) were used in this study. The control diet contained 12% of the total calories, which was supplied from corn oil. The HF diet contains 60% of total calories, which is supplied from corn oil, oleic acid, and the saturated fatty acids palmitic and stearic. Animals were grouped into 4 groups and studied for 42 days, including rats fed with 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv) and HF+100mg/kg body weight of carvone (100 Carv). After 42 days, 3 animals per group were fasted16-18 h and killed, and blood and liver samples were obtained to evaluate the biochemical analyses. In addition, animals were weighed at the initial and end of the trial for body changes. To evaluate the regression studies, animals were first fed the HF diet for another 42 days up to induce hepatic steatosis. A number of seven animals in each group were randomized and fed either the HF diet or HF+100mg/kg body weight of carvone (100 Carv).
Biochemical Analysis
Samples from total liver lipids were extracted, and TBARS, triglycerides, and cholesterol were evaluated as reported by others [18]. The serum concentrations of nonesterified fatty acid (NEFA), triglycerides, and cholesterol were evaluated using commercial Kits (Abacam).
Statistical Analysis
The results are reported as mean±SD. To compare the data, a one-way ANOVA and Turkey’s post-test were applied.
Findings
Effects of different levels of carvone on liver and body weight are shown in Figure 1. Results showed that rats in the HF group showed higher body and liver weight than the control group (p<0.05). Rats fed with HF diets containing 50 and 100mg/kg of carvone showed lower weights than those of the HF group (p<0.05).
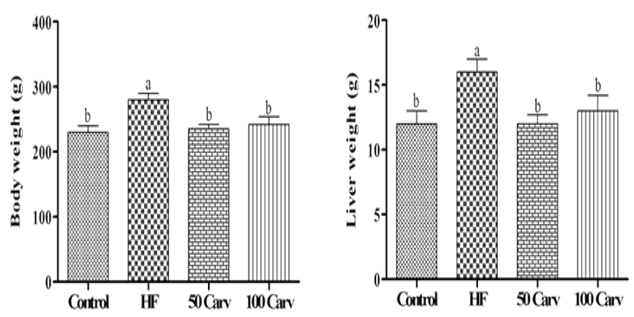
Figure 1. Effect of carvone on body weight and liver weight. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6wk. Superscripts show significant differences at p<0.05
Effects of carvone on serum and liver levels of triglycerides and cholesterol are shown in Figure 2. Results showed that rats fed with an HF diet showed higher levels of triglyceride and cholesterol compared to the control group (p<0.05). Dietary inclusion of carvone, especially in the higher levels, could reverse the effects of HF on cholesterol and triglycerides (p<0.05).
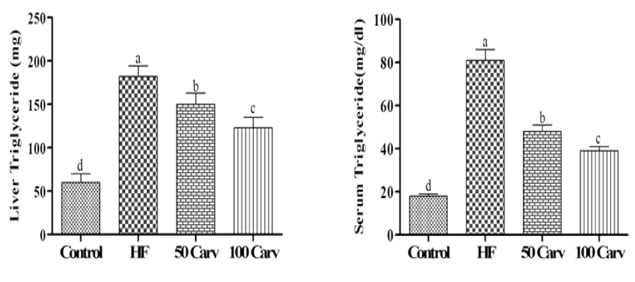
Figure 2. Effect of carvone on serum and liver triglycerides. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6wk. Data were reported as means±SD. Superscripts show significant differences at p<0.05
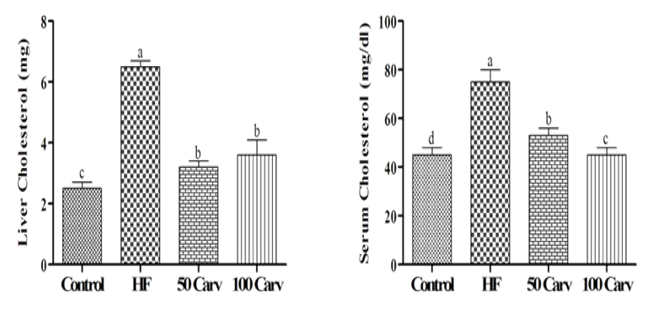
Figure 3. Effect of carvone on serum and liver cholesterol. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6wk. Data were reported as means±SD. Superscripts show significant differences at p<0.05
The effects of carvone on liver TBARS are illustrated in Figure 4. Results showed that lipid peroxidation was significantly higher in the HF group compared with the control group (p<0.05). Carvone supplementing could significantly alleviate the adverse effects of HF (p<0.05), and the best response was observed in 100mg/kg of the carvone.
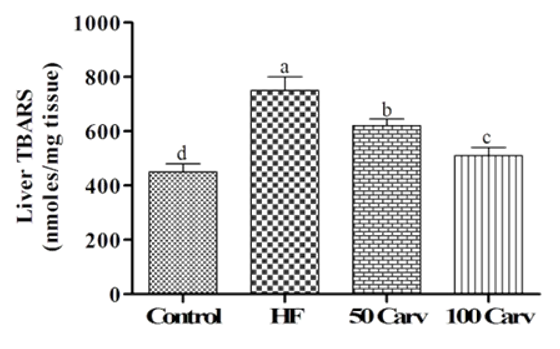
Figure 4. Effect of carvone on liver lipid peroxidation products. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6 wk. Data were reported as means±SD. Superscripts show significant differences at p<0.05.
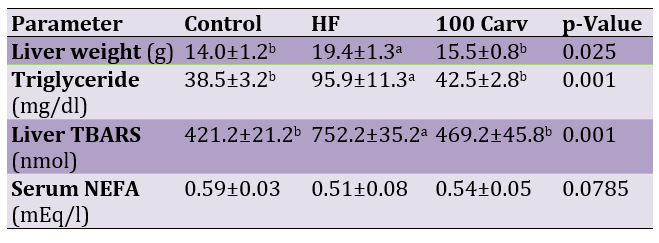
Following the induction of hepatic steatosis, liver weight and the serum concentrations of triglycerides and TBARS were significantly higher in the HF group in comparison to the control group (p<0.05; Table 1), and the use of carvone alleviated the effects of HF (p<0.05). Carvone did not have effects on serum NEFA (p<0.05).
Table 1. Effects of carvone on liver weight and some serum parameters
Discussion
With regards to previous studies, animals fed with HF diets (50-75% calories derived from fat), 60% in the current present, progress hepatic steatosis and signs of initial NASH related to dyslipidemia, insulin resistance, and changes in mitochondria which result in the increased oxidative stress [19-21]. However, HF diets cannot significantly develop severe steatohepatitis, but their pathophysiology resembles human NAFLD [19-21]. The HF diet-induced NAFLD model is commonly used to evaluate the pathogenesis of NAFLD in order to find treatment strategies [22-24]. However, the use of carvone could alleviate the adverse effects of NAFLD. In addition to investigating steatosis, we also showed that carvone can regress preexisting steatosis, which could be found in clinical conditions in humans. Our results show that supplementation with carvone regresses preexisting hepatic steatosis as assessed by biochemical liver triglyceride contents. Results also showed that carvone could significantly reduce body and liver weights. With regards to previous studies, it is also recommended to use the treatment strategies for weight loss, which improve hepatic steatosis [25-28]. In summary, carvone reduces the levels of cholesterol and triglycerides in the liver. It could be stated that the decrease in both triglycerides and cholesterol in the liver causes an increase in liver weight, and reduced lipids may be the reason for the reduction in liver weight. Since both levels of carvone could improve the parameters and similar effects were observed in both groups, it is possible that carvone in lower doses can inhibit hepatic steatosis. We believe that carvone improves levels of triglycerides and cholesterol by an antioxidant mechanism, reducing cholesterol and triglycerides and reducing liver weight. The data approved our findings for the antioxidant properties of carvone for TBARS, which was significantly lower in carvone groups. A study reported the antioxidant effect of D-carvone on DPPH• and ABTS+; d-carvone showed concentration-dependent antioxidant potential [28].
Conclusion
Carvone inhibited and reversed hepatic steatosis. The clinical progression of carvone formulations and carvone-related structures for the treatment of NAFLD will be significant in preparing for the need to develop therapeutic agents for NAFLD/NASH and other forms of fatty liver disease.
Acknowledgments: None declared by the authors.
Ethical Permission: The Institute for Phytochemical Research (IPR), Germany, approved this study.
Conflicts of Interests: None declared by the authors.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Nonalcoholic fatty liver disease (NAFLD) is a condition in which fat significantly aggregates in the liver of a patient who lacks a history of alcohol abuse [1].
NAFLD is grouped into two groups, including simple steatosis and nonalcoholic steatohepatitis (NASH). Under NASH conditions, steatosis, intralobular inflammation, and hepatocellular are found in progressive fibrosis [2]. Permanent NASH may be caused by liver cirrhosis and hepatocellular carcinoma [3-5]. NAFLD not only increases the risk of developing liver disease but is also one key component for metabolic syndrome, obesity, and type 2 diabetes [6]. NAFLD encompasses a broad range, including simple fatty liver (intracellular lipids >5%) up to progressive NASH, which is accompanied by lobular inflammation, fibrosis, and cirrhosis and increases the risk for hepatocellular carcinoma [7]. Increased triglyceride aggregation in hepatocytes indicates NAFLD, which is severely related to hepatic insulin resistance [8]. Increased triglyceride formation is observed in fatty livers accompanied by obesity and type 2 diabetes mellitus [9]. Hepatic fat aggregation causes hepatic insulin resistance by promoting gluconeogenesis and activating the PKC-ε and JNK1 signaling pathways [10]. It is not approved agents available for the treatment of NAFLD. Improvement of some factors, such as weight reduction and dietary fat intake, are usually known as treatment modalities in NAFLD disease [11]. Studies showed that insulin sensitizers, such as thiazolidinediones, and some antioxidants could improve clinical conditions of NASH [12, 13]. Carvone (5-isopropenyl-2-methyl-2-cyclohexenone) is one monocyclic monoterpene ketone that is found in 70 different plants. It is one of the main components of caraway oil. It is known to have biological activities, including antimicrobial [14], nematicidal [15], antitumor [16], and antioxidant [17] properties. It seems that carvone could improve and prevent hepatic steatosis in rat models of NAFLD due to its antioxidant properties. This study was thus conducted to evaluate the effects of carvone on hepatic steatosis and NAFLD by preventing hepatic triglyceride formation and oxidative processes.
Materials and Methods
Materials
Carvone was purchased from Sigma Chemicals Company, St. Louis, MO, USA, and kept at 2-4°C and protected from sunlight. Other chemicals were purchased from commercial suppliers.
Animal studies
A total number of 60 Wistar rats (180±10g) were used in this study. The control diet contained 12% of the total calories, which was supplied from corn oil. The HF diet contains 60% of total calories, which is supplied from corn oil, oleic acid, and the saturated fatty acids palmitic and stearic. Animals were grouped into 4 groups and studied for 42 days, including rats fed with 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv) and HF+100mg/kg body weight of carvone (100 Carv). After 42 days, 3 animals per group were fasted16-18 h and killed, and blood and liver samples were obtained to evaluate the biochemical analyses. In addition, animals were weighed at the initial and end of the trial for body changes. To evaluate the regression studies, animals were first fed the HF diet for another 42 days up to induce hepatic steatosis. A number of seven animals in each group were randomized and fed either the HF diet or HF+100mg/kg body weight of carvone (100 Carv).
Biochemical Analysis
Samples from total liver lipids were extracted, and TBARS, triglycerides, and cholesterol were evaluated as reported by others [18]. The serum concentrations of nonesterified fatty acid (NEFA), triglycerides, and cholesterol were evaluated using commercial Kits (Abacam).
Statistical Analysis
The results are reported as mean±SD. To compare the data, a one-way ANOVA and Turkey’s post-test were applied.
Findings
Effects of different levels of carvone on liver and body weight are shown in Figure 1. Results showed that rats in the HF group showed higher body and liver weight than the control group (p<0.05). Rats fed with HF diets containing 50 and 100mg/kg of carvone showed lower weights than those of the HF group (p<0.05).

Figure 1. Effect of carvone on body weight and liver weight. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6wk. Superscripts show significant differences at p<0.05
Effects of carvone on serum and liver levels of triglycerides and cholesterol are shown in Figure 2. Results showed that rats fed with an HF diet showed higher levels of triglyceride and cholesterol compared to the control group (p<0.05). Dietary inclusion of carvone, especially in the higher levels, could reverse the effects of HF on cholesterol and triglycerides (p<0.05).

Figure 2. Effect of carvone on serum and liver triglycerides. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6wk. Data were reported as means±SD. Superscripts show significant differences at p<0.05

Figure 3. Effect of carvone on serum and liver cholesterol. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6wk. Data were reported as means±SD. Superscripts show significant differences at p<0.05
The effects of carvone on liver TBARS are illustrated in Figure 4. Results showed that lipid peroxidation was significantly higher in the HF group compared with the control group (p<0.05). Carvone supplementing could significantly alleviate the adverse effects of HF (p<0.05), and the best response was observed in 100mg/kg of the carvone.

Figure 4. Effect of carvone on liver lipid peroxidation products. Animals were fed 1) control diet (Control), 2) HF diet (HF), 3) HF+50mg/kg body weight of carvone (50 Carv), and HF+100mg/kg body weight of carvone (100 Carv) for 6 wk. Data were reported as means±SD. Superscripts show significant differences at p<0.05.
Following the induction of hepatic steatosis, liver weight and the serum concentrations of triglycerides and TBARS were significantly higher in the HF group in comparison to the control group (p<0.05; Table 1), and the use of carvone alleviated the effects of HF (p<0.05). Carvone did not have effects on serum NEFA (p<0.05).
Table 1. Effects of carvone on liver weight and some serum parameters

Discussion
With regards to previous studies, animals fed with HF diets (50-75% calories derived from fat), 60% in the current present, progress hepatic steatosis and signs of initial NASH related to dyslipidemia, insulin resistance, and changes in mitochondria which result in the increased oxidative stress [19-21]. However, HF diets cannot significantly develop severe steatohepatitis, but their pathophysiology resembles human NAFLD [19-21]. The HF diet-induced NAFLD model is commonly used to evaluate the pathogenesis of NAFLD in order to find treatment strategies [22-24]. However, the use of carvone could alleviate the adverse effects of NAFLD. In addition to investigating steatosis, we also showed that carvone can regress preexisting steatosis, which could be found in clinical conditions in humans. Our results show that supplementation with carvone regresses preexisting hepatic steatosis as assessed by biochemical liver triglyceride contents. Results also showed that carvone could significantly reduce body and liver weights. With regards to previous studies, it is also recommended to use the treatment strategies for weight loss, which improve hepatic steatosis [25-28]. In summary, carvone reduces the levels of cholesterol and triglycerides in the liver. It could be stated that the decrease in both triglycerides and cholesterol in the liver causes an increase in liver weight, and reduced lipids may be the reason for the reduction in liver weight. Since both levels of carvone could improve the parameters and similar effects were observed in both groups, it is possible that carvone in lower doses can inhibit hepatic steatosis. We believe that carvone improves levels of triglycerides and cholesterol by an antioxidant mechanism, reducing cholesterol and triglycerides and reducing liver weight. The data approved our findings for the antioxidant properties of carvone for TBARS, which was significantly lower in carvone groups. A study reported the antioxidant effect of D-carvone on DPPH• and ABTS+; d-carvone showed concentration-dependent antioxidant potential [28].
Conclusion
Carvone inhibited and reversed hepatic steatosis. The clinical progression of carvone formulations and carvone-related structures for the treatment of NAFLD will be significant in preparing for the need to develop therapeutic agents for NAFLD/NASH and other forms of fatty liver disease.
Acknowledgments: None declared by the authors.
Ethical Permission: The Institute for Phytochemical Research (IPR), Germany, approved this study.
Conflicts of Interests: None declared by the authors.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16): 1221-31. [Link] [DOI:10.1056/NEJMra011775]
2. Takahashi Y, Fukusato T. Pathology of nonalcoholic steatohepatitis. In: Current research in hepatology 2. Trivandrum: Research Media. 2008:99-112. [Link]
3. Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: A follow-up study of forty-two patients for up to 21 years. Hepatol. 1990;11(1):74-80. [Link] [DOI:10.1002/hep.1840110114]
4. Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: A clinical histopathological study. Am J Gastroenterol. 2003;98(9): 2042-7. [Link] [DOI:10.1111/j.1572-0241.2003.07659.x]
5. Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: Old questions and new insights. Science. 2011;332(6037): 1519-23. [Link] [DOI:10.1126/science.1204265]
6. Misra VL, Khashab M, Chalasani N. Non-alcoholic fatty liver disease and cardiovascular risk. Curr Opin Gastroenterol Rep. 2009;11(1): 50-5. [Link] [DOI:10.1007/s11894-009-0008-4]
7. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterol. 2002;122(6): 1649-57. [Link] [DOI:10.1053/gast.2002.33573]
8. Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118(3): 829-38. [Link] [DOI:10.1172/JCI34275]
9. Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, et al. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: Further evidence for an etiologic association. Hepatology. 2002;35(2): 367-72. [Link] [DOI:10.1053/jhep.2002.30690]
10. Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279(31): 32345-53. [Link] [DOI:10.1074/jbc.M313478200]
11. Ahmed MH, Byrne CD. Current treatment of non-alcoholic fatty liver disease. Diabetes Obes Metab. 2009;11:188-95. [Link] [DOI:10.1111/j.1463-1326.2008.00926.x]
12. Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96(2):519-25. [Link] [DOI:10.1111/j.1572-0241.2001.03553.x]
13. Harrison SA, Torgerson S, HayashiP, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98(11):2485-90. [Link] [DOI:10.1111/j.1572-0241.2003.08699.x]
14. Farag RS, Daw ZY, Abo-Raya SH. Influence of some spice essential oils on Aspergilrus pamsiticus growth and production of aflatoxin in a synthetic medium. J Food Sci. 1989;54(1):74-6. [Link] [DOI:10.1111/j.1365-2621.1989.tb08571.x]
15. Saxena DB, Goswami BK, Tomar SS. Nematicidal activity of some essential oils against Meloidogyne incognita. Indian Perfum. 1987;31:150-4. [Link]
16. Zheng GQ, Kenney PM, Lam LK. Effects of carvone compounds on glutathion S-transferase activity in A/J mice. J Agric Food Chem. 1992;40:751-5. [Link] [DOI:10.1021/jf00017a008]
17. Johri RK. Cuminum cyminum and Carumcarvi: An update. Pharmacogn Rev. 2011;5(9):63-72. [Link] [DOI:10.4103/0973-7847.79101]
18. Ganji SH, Kukes GD, Lambrecht N, Kashyap ML, Kamanna VS. Therapeutic role of niacin in the prevention and regression of hepatic steatosis in rat model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2014;306(4):G320-7. [Link] [DOI:10.1152/ajpgi.00181.2013]
19. Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87(1):1-16. [Link] [DOI:10.1111/j.0959-9673.2006.00465.x]
20. Deng QG, She H, Cheng JH, French SW, Koop DR, Xiong S, et al. Steatohepatitis induced by intragastric overfeeding in mice. Hepatol. 2005;42(4):905-14. [Link] [DOI:10.1002/hep.20877]
21. Schattenberg JM, Galle PR. Animal models of non-alcoholic steatohepatitis: Of mice and man. Dig Dis. 2010;28(1):247-54. [Link] [DOI:10.1159/000282097]
22. Carmiel-Haggai M, Cederbaum AI, Nieto N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005;19(1):136-8. [Link] [DOI:10.1096/fj.04-2291fje]
23. Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverse diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282(31):22678-88. [Link] [DOI:10.1074/jbc.M704213200]
24. Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemiain obese mice. Hepatol. 2005;42(2):362-71. [Link] [DOI:10.1002/hep.20783]
25. Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006;82(967):315-22. [Link] [DOI:10.1136/pgmj.2005.042200]
26. Jin YJ, Kim KM, Hwang S, Lee SG, Ha TY, Song GW, et al. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: Analysis of biopsies of living liver donors. J Gastroenterol Hepatol. 2012;27(8):1341-7. [Link] [DOI:10.1111/j.1440-1746.2012.07165.x]
27. Ueno T, Sugawara H, Sujaku K, Hashimoto O, Tsuji R, Tamaki S,et al. Therapeutic effects ofrestricted diet and exercise in obese patients with fatty liver. J Hepatol. 1997;27(1):103-7. [Link] [DOI:10.1016/S0168-8278(97)80287-5]
28. Rajeshwari T, Raja B. Antioxidant and free radical scavenging effect of D-carvone in hypertensive rats. In Vivo and In Vitro study. Int Lett Nat Sci. 2015;35:6-12. [Link] [DOI:10.18052/www.scipress.com/ILNS.35.6]









