GMJ Medicine
eISSN : 2626-3041
Volume 4, Issue 1 (2025)
GMJM 2025, 4(1): 7-11 |
Back to browse issues page
History
Received: 2024/07/15 | Accepted: 2024/12/28 | Published: 2025/02/12
Received: 2024/07/15 | Accepted: 2024/12/28 | Published: 2025/02/12
How to cite this article
Rabinovich A, Romanoff N, Mordvinov D, Ivanov M. Effects of Oral Administration of Pulegone on Carbon Tetrachloride-Induced Oxidative Stress in Wistar Rats. GMJM 2025; 4 (1) :7-11
URL: http://gmedicine.de/article-2-243-en.html
URL: http://gmedicine.de/article-2-243-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Pirogov Russian National Research Medical University, Moscow, Russia
2- Institute for Biomedical Research, Association of Pharmaceutical Research and Development, Kyiv, Ukraine
2- Institute for Biomedical Research, Association of Pharmaceutical Research and Development, Kyiv, Ukraine
Keywords:
| Abstract (HTML) (2455 Views)
Full-Text: (1098 Views)
Introduction
Carbon tetrachloride, CCl4 is usually applied to induce the toxicity and hepatic fibrosis. The hepatotoxic effect of CCl4 could be attributed to its fast cleavage through cytochrome P450 in hepatocytes, that produces trichloromethyl radicals leading to lipid peroxidation and then to membrane injury [1].
Activation of the cytochrome catabolizes CCl4 to trichloromethyl radical (CCl3) which produces toxic reactive trichloromethyl peroxy radical [2]. Oxidative stress increases in case of imbalance between formation and scavenging of reactive oxygen species (ROS) [2]. Oxidative stress is known as cause for the different degenerative diseases such as oxidative stress [3]. Natural antioxidants are known as efficient and safe treatments for hepatotoxicity compared with synthetic antioxidants [4, 5].
In this connection, Pulegone is a natural monoterpene ketone which is contained in the essential oil of a class of plants including Mentha species. It is reported that Mentha pulegium L. and Mentha longifolia L. have 60%–90% and 17% pulegone, respectively, as main component and also have anti-bacterial, antioxidant and anti-inflammatory properties [6, 7]. It is known to have antimicrobial activity, especially against all the Salmonella species [8].
A study showed that major volatile compounds of Satureja macrostema such as pulegone showed antioxidant activity in 2, 2-diphenyl-1-picrylhydrazyl and 2, 2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid [9]. It seems that pulegone could alleviate adverse effects of oxidative stress in carbon tetrachloride-induced oxidative stress. Thus, this study, for first time, was conducted to evaluate the effects of oral administration of pulegone in carbon tetrachloride-induced oxidative stress in Wistar rats.
Materials and Methods
Animals
A total of 20 adult Male Wistar rats with initial weight of 220±20g, were used in this study. Animals had allowed to ad libitum to a pelleted diet and water in 25±2°C with a 12-h light/dark cycle. We did not use female rats to avoid the data variability obtained by hormonal cycles in females.
Experimental procedure
The Pulegone was procured from Sigma Chemical Co, USA. Based on previous acute toxicological reports of pulegone, 20mg/kg body weight of pulegone was prepared to be nontoxic for rats [10]. The levels of 20 and 30mg/kg doses of pulegone were used. Twenty rats were randomly allocated into four groups and five animals in per group and pulegone was gavaged with animal oral feeding cannula and divided as follows:
Group 1: control- olive oil (1ml/kg), (Control),
Group 2: toxic control-30% CCl4, (Toxic),
Group 3: Pulegone 20mg/kg + 30% CCl4, (Pulegone-20),
Group 4: Pulegone 30mg/kg + 30% CCl4, (Pulegone-30).
Rats in Groups 2, 3, and 4 were treated with 30% CCl4 olive oil (1mL/Kg) by using intra-peritoneal administration for one time every three days for eight-week period [2]. Animals in pulegone groups received 20mg/kg and 30mg/kg pulegone, 72h prior to CCl4 treatment. In the end of trial, experimental animals were anesthetized and sacrificed by cervical dislocation, and the liver samples were collected, rinsed with saline solution, and stored at -80°C.
Lipid peroxidation, lipid profile, enzymatic and non-enzymatic antioxidants
Malondialdehyde (MDA) was assessed as one standard procedure for lipid peroxidation in the 532 nm as reported by others [11]. We have used Chloroform-methanol mixture (2:1 v/v) [12] for extraction of lipids in the liver tissue for investigation of the total cholesterol [13], triacylglycerides [14, 15], and phospholipids [16]. Standard methods were applied to estimation of the superoxide dismutase (SOD) [17], catalase (CAT) [18], reduced glutathione (GSH), glutathione peroxidase (GPx) [19], Vitamin C [20] and vitamin E [21].
Statistical analysis
The data were analyzed by one-way Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test (DMRT). SPSS 23 software was used for analysis of the data and Graph Pad Prism was used for analysis of the data.
Findings
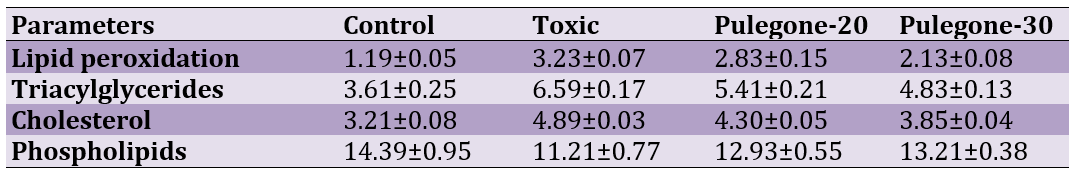
Effects of the different levels of pulegone on lipid peroxidation, triacylglycerides, cholesterol and phospholipids in liver of rats are shown in Table 1.
Table 1. Effects of the different levels of pulegoneon on lipid peroxidation (nmoles of MDA/mg protein), Triacylglycerides (mg/g tissue wt), cholesterol (mg/g tissue wt) and phospholipids (mg/g tissue wt) in liver of Wistar rats
Carbon tetrachloride, CCl4 is usually applied to induce the toxicity and hepatic fibrosis. The hepatotoxic effect of CCl4 could be attributed to its fast cleavage through cytochrome P450 in hepatocytes, that produces trichloromethyl radicals leading to lipid peroxidation and then to membrane injury [1].
Activation of the cytochrome catabolizes CCl4 to trichloromethyl radical (CCl3) which produces toxic reactive trichloromethyl peroxy radical [2]. Oxidative stress increases in case of imbalance between formation and scavenging of reactive oxygen species (ROS) [2]. Oxidative stress is known as cause for the different degenerative diseases such as oxidative stress [3]. Natural antioxidants are known as efficient and safe treatments for hepatotoxicity compared with synthetic antioxidants [4, 5].
In this connection, Pulegone is a natural monoterpene ketone which is contained in the essential oil of a class of plants including Mentha species. It is reported that Mentha pulegium L. and Mentha longifolia L. have 60%–90% and 17% pulegone, respectively, as main component and also have anti-bacterial, antioxidant and anti-inflammatory properties [6, 7]. It is known to have antimicrobial activity, especially against all the Salmonella species [8].
A study showed that major volatile compounds of Satureja macrostema such as pulegone showed antioxidant activity in 2, 2-diphenyl-1-picrylhydrazyl and 2, 2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid [9]. It seems that pulegone could alleviate adverse effects of oxidative stress in carbon tetrachloride-induced oxidative stress. Thus, this study, for first time, was conducted to evaluate the effects of oral administration of pulegone in carbon tetrachloride-induced oxidative stress in Wistar rats.
Materials and Methods
Animals
A total of 20 adult Male Wistar rats with initial weight of 220±20g, were used in this study. Animals had allowed to ad libitum to a pelleted diet and water in 25±2°C with a 12-h light/dark cycle. We did not use female rats to avoid the data variability obtained by hormonal cycles in females.
Experimental procedure
The Pulegone was procured from Sigma Chemical Co, USA. Based on previous acute toxicological reports of pulegone, 20mg/kg body weight of pulegone was prepared to be nontoxic for rats [10]. The levels of 20 and 30mg/kg doses of pulegone were used. Twenty rats were randomly allocated into four groups and five animals in per group and pulegone was gavaged with animal oral feeding cannula and divided as follows:
Group 1: control- olive oil (1ml/kg), (Control),
Group 2: toxic control-30% CCl4, (Toxic),
Group 3: Pulegone 20mg/kg + 30% CCl4, (Pulegone-20),
Group 4: Pulegone 30mg/kg + 30% CCl4, (Pulegone-30).
Rats in Groups 2, 3, and 4 were treated with 30% CCl4 olive oil (1mL/Kg) by using intra-peritoneal administration for one time every three days for eight-week period [2]. Animals in pulegone groups received 20mg/kg and 30mg/kg pulegone, 72h prior to CCl4 treatment. In the end of trial, experimental animals were anesthetized and sacrificed by cervical dislocation, and the liver samples were collected, rinsed with saline solution, and stored at -80°C.
Lipid peroxidation, lipid profile, enzymatic and non-enzymatic antioxidants
Malondialdehyde (MDA) was assessed as one standard procedure for lipid peroxidation in the 532 nm as reported by others [11]. We have used Chloroform-methanol mixture (2:1 v/v) [12] for extraction of lipids in the liver tissue for investigation of the total cholesterol [13], triacylglycerides [14, 15], and phospholipids [16]. Standard methods were applied to estimation of the superoxide dismutase (SOD) [17], catalase (CAT) [18], reduced glutathione (GSH), glutathione peroxidase (GPx) [19], Vitamin C [20] and vitamin E [21].
Statistical analysis
The data were analyzed by one-way Analysis of Variance (ANOVA) and Duncan’s Multiple Range Test (DMRT). SPSS 23 software was used for analysis of the data and Graph Pad Prism was used for analysis of the data.
Findings
Effects of the different levels of pulegone on lipid peroxidation, triacylglycerides, cholesterol and phospholipids in liver of rats are shown in Table 1.
Table 1. Effects of the different levels of pulegoneon on lipid peroxidation (nmoles of MDA/mg protein), Triacylglycerides (mg/g tissue wt), cholesterol (mg/g tissue wt) and phospholipids (mg/g tissue wt) in liver of Wistar rats

Results showed that levels of lipid peroxidation, triacylglycerides and cholesterol were significantly higher and also level of phospholipids was significantly lower in Toxic group compared with control group (p<0.05). Oral administration of pulegone reversed adverse effects of CCl4 on the
mentioned parameters (p<0.05). The best response was observed in level of 30 mg/kg pulegone. The data for enzymatic and non-enzymatic antioxidants are illustrated in Figure 1.
As results show, CCl4 lowered levels of antioxidants, but pulegone could partly spare antioxidants and the best response was observed in the highest level (p<0.05).
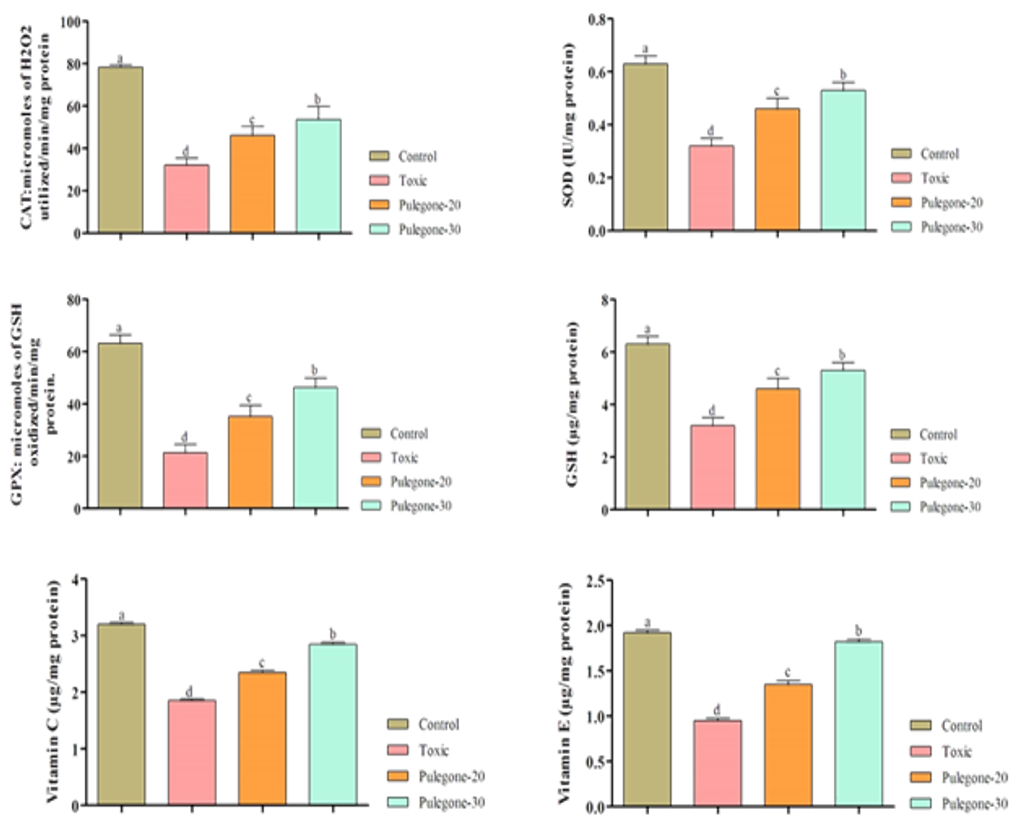
Figure 1. Effects of the different levels of pulegone on enzymatic and non-enzymatic antioxidants in liver of rats. Superscripts (a-d) show significant differences among groups
Discussion
The liver is known to have essential roles in metabolic homeostasis and it is also responsible for the metabolism, synthesis, storage and redistribution of nutrients and macromolecules. It is not only involved in the metabolism and detoxification of the body but also removes wastes and xenobiotics via metabolic conversion and biliary excretion [22]. The CCl4 metabolism has been used as model for liver necrosis and fibrosis. Oxidative stress is known to have essential role in CCl4-induced toxicity. Moreover, the induction of trichloromethyl-free radical increases lipid peroxidation process. It destroys membrane integrity and Ca2+ homeostasis for production of hepatocellular damage [23]. In the current study, the CCl4 disturbed lipid profile and increased lipid oxidation and decreased levels of antioxidants. However, the use of pulegone could attenuate the effects of CCl4 which could be attributed to its antioxidant activity. It is shown that antioxidant phyto-chemicals protect oxidative damages [24]. Natural antioxidants are involved in oxidative stress by ROS scavenging activity and induction of antioxidant and phase II detoxifying enzymes [25]. Improvement in lipids levels in pulegone could be attributed to CCl4 mechanism. The CCl4-induced liver injury stimulates lipid oxidation and increases production of ROS [23]. Our findings for lipid oxidation showed higher levels in Toxic group in comparison to control group in terms of MDA, as final-product of membrane lipid oxidation. It means that increased lipid oxidation is paralleled decreased antioxidant enzymes, as illustrated in Figure 1. It seems that pulegone spares antioxidants and help to protect of the antioxidants. On the other hand, we observed a raised level in cholesterol and triacylglycerides in Toxic group. It is accepted that CCl4 increases levels of cholesterol to rise in hepatocytes. On the other hand, reduced levels of phospholipids were seen following administration of CCl4 treatment, that could be a result to an augmentation in phospholipase activity [26, 27]. To better understand of the pulegone activities, it is need to explain further the antioxidant parameters. GSH is one key non-enzymatic antioxidant which controls the intracellular redox homeostasis and protects cells from deleterious effects of ROS [28]. CCl4 reduced levels of GSH but pulegone spared its levels. To be higher GSH levels can protect the liver against oxidative damage via directly scavenging the ROS by component of the GSH redox system including GPx, glutathione reductase, and glutathione-s-transferase [29].
On the other hand, results showed that CCl4 reduced levels of vitamins C and E. Increased levels of vitamins means antioxidant nature of pulegone. It seems that treatment with pulegone maintains membrane from damages and also protects active forms of vitamin C and vitamin E from ROS by increased levels of GSH, due to their close relation [30].
Three main enzymes CAT, GPx, and SOD are initial lines against damages. SOD is known to have ability for conversion of the superoxide anion to H2O2 and O2. The H2O2 decreased is catalyzed via CAT and GPx that protects the tissue from biomolecule-damaging ROS [31]. Thus, CCl4 reduces the levels of enzymatic and non-enzymatic antioxidants but pulegone attenuates its effects.
Conclusion
The CCl4 has adverse effects on liver lipids and increases levels lipid oxidation and also lowers the levels of antioxidants. Administration of pulegone alleviated the effects of CCl4 on liver lipids and antioxidants. Pulegone spares the antioxidants and helps to protect against damages and thus reduces levels of lipids.
Acknowledgements: None declared by the authors.
Ethical Permission: Approval for this study was obtained from Association of Pharmaceutical Research and Development in Kyiv.
Conflicts of Interests: None declared by the authors.
Authors' contributions: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
As results show, CCl4 lowered levels of antioxidants, but pulegone could partly spare antioxidants and the best response was observed in the highest level (p<0.05).

Figure 1. Effects of the different levels of pulegone on enzymatic and non-enzymatic antioxidants in liver of rats. Superscripts (a-d) show significant differences among groups
Discussion
The liver is known to have essential roles in metabolic homeostasis and it is also responsible for the metabolism, synthesis, storage and redistribution of nutrients and macromolecules. It is not only involved in the metabolism and detoxification of the body but also removes wastes and xenobiotics via metabolic conversion and biliary excretion [22]. The CCl4 metabolism has been used as model for liver necrosis and fibrosis. Oxidative stress is known to have essential role in CCl4-induced toxicity. Moreover, the induction of trichloromethyl-free radical increases lipid peroxidation process. It destroys membrane integrity and Ca2+ homeostasis for production of hepatocellular damage [23]. In the current study, the CCl4 disturbed lipid profile and increased lipid oxidation and decreased levels of antioxidants. However, the use of pulegone could attenuate the effects of CCl4 which could be attributed to its antioxidant activity. It is shown that antioxidant phyto-chemicals protect oxidative damages [24]. Natural antioxidants are involved in oxidative stress by ROS scavenging activity and induction of antioxidant and phase II detoxifying enzymes [25]. Improvement in lipids levels in pulegone could be attributed to CCl4 mechanism. The CCl4-induced liver injury stimulates lipid oxidation and increases production of ROS [23]. Our findings for lipid oxidation showed higher levels in Toxic group in comparison to control group in terms of MDA, as final-product of membrane lipid oxidation. It means that increased lipid oxidation is paralleled decreased antioxidant enzymes, as illustrated in Figure 1. It seems that pulegone spares antioxidants and help to protect of the antioxidants. On the other hand, we observed a raised level in cholesterol and triacylglycerides in Toxic group. It is accepted that CCl4 increases levels of cholesterol to rise in hepatocytes. On the other hand, reduced levels of phospholipids were seen following administration of CCl4 treatment, that could be a result to an augmentation in phospholipase activity [26, 27]. To better understand of the pulegone activities, it is need to explain further the antioxidant parameters. GSH is one key non-enzymatic antioxidant which controls the intracellular redox homeostasis and protects cells from deleterious effects of ROS [28]. CCl4 reduced levels of GSH but pulegone spared its levels. To be higher GSH levels can protect the liver against oxidative damage via directly scavenging the ROS by component of the GSH redox system including GPx, glutathione reductase, and glutathione-s-transferase [29].
On the other hand, results showed that CCl4 reduced levels of vitamins C and E. Increased levels of vitamins means antioxidant nature of pulegone. It seems that treatment with pulegone maintains membrane from damages and also protects active forms of vitamin C and vitamin E from ROS by increased levels of GSH, due to their close relation [30].
Three main enzymes CAT, GPx, and SOD are initial lines against damages. SOD is known to have ability for conversion of the superoxide anion to H2O2 and O2. The H2O2 decreased is catalyzed via CAT and GPx that protects the tissue from biomolecule-damaging ROS [31]. Thus, CCl4 reduces the levels of enzymatic and non-enzymatic antioxidants but pulegone attenuates its effects.
Conclusion
The CCl4 has adverse effects on liver lipids and increases levels lipid oxidation and also lowers the levels of antioxidants. Administration of pulegone alleviated the effects of CCl4 on liver lipids and antioxidants. Pulegone spares the antioxidants and helps to protect against damages and thus reduces levels of lipids.
Acknowledgements: None declared by the authors.
Ethical Permission: Approval for this study was obtained from Association of Pharmaceutical Research and Development in Kyiv.
Conflicts of Interests: None declared by the authors.
Authors' contributions: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. El-Boshy ME, Abdelhamidb F, Richab E, AshshiaA, Gaitha M, Qustya N. Attenuation of CCl4 induced oxidative stress, immunosuppressive, hepatorenal damage by Fucoidan in rats. J Clin Toxicol. 2017;7(3):1-7. [Link] [DOI:10.4172/2167-7972.1000348]
2. Goodla L, Manubolu MH, Pathakoti K, Jayakumar T, Sheu J, Fraker M, Tchounwou PB, et al. Protective effects of ammannia baccifera against CCl4induced oxidative stress in rats. Int J Environ Res Public Health. 2019;16(8):1440. [Link] [DOI:10.3390/ijerph16081440]
3. Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28(10):1456-62. [Link] [DOI:10.1016/S0891-5849(00)00252-5]
4. Kadri A, Zarai Z, Ben Chobba I, Bekir A, Gharsallah N, DamakM, et al. Chemical composition and antioxidant activity of Marrubium vulgare L. Essential oil from Tunisia. Afr J Biotechnol. 2011;10(19):3908-14. [Link]
5. Lavanya G, Voravuthikunchai SP, Towatana NH. Acetone extract from Rhodomyrtus tomentosa: A potent natural antioxidant. Evid Based Complementary Altern Med. 2012;2012:535479. [Link] [DOI:10.1155/2012/535479]
6. Jalilzadeh Amin G, Maham M, Dalir Naghadeh B, Kheiri F. Effects of Mentha longifolia essential oil on ruminal and abomasal longitudinal smooth muscle in sheep. J Essent Oil Res. 2012;24:61-9. [Link] [DOI:10.1080/10412905.2012.646019]
7. Turner GW, Croteau R. Organization of monoterpene biosynthesis in Mentha. Immunocytochemical localizations of geranyl diphosphate synthase, limonene-6-hydroxylase, isopiperitenol dehydrogenase, and pulegone reductase. Plant Physiol. 2004;136(4):4215-27. [Link] [DOI:10.1104/pp.104.050229]
8. Flamini G, Cioni PL, Puleio R, Morelli I, Panizzi L. Antimicrobial activity of the essential oil of Calamintha nepeta and its constituent pulegone against bacteria and fungi. Phytother Res. 1999;13(4):349-51.
https://doi.org/10.1002/(SICI)1099-1573(199906)13:4<349::AID-PTR446>3.0.CO;2-Z [Link] [DOI:10.1002/(SICI)1099-1573(199906)13:43.0.CO;2-Z]
9. Torres-Martínez R, García-Rodríguez YM, Ríos-Chávez P, Saavedra-Molina A, López-Meza JE, Ochoa-Zarzosa A, et al. Antioxidant activity of the essential oil and its major terpenes of Satureja macrostema (Moc. and Sessé ex Benth.) Briq. Pharmacogn Mag. 2017;13(4):S875-80. [Link]
10. Cheraghali Z, Mohammadi R, Jalilzadeh-amin G. Planimetric and biomechanical study of local effect of pulegone on full thickness wound healing in rat. Malays J Med Sci. 2017;24(5):52-61. [Link] [DOI:10.21315/mjms2017.24.5.6]
11. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351-8. [Link] [DOI:10.1016/0003-2697(79)90738-3]
12. Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497-509. [Link] [DOI:10.1016/S0021-9258(18)64849-5]
13. Parekh AC, Jung DH. Cholesterol determination with ferric acetate-uranium acetate and sulfuric acid-ferrous sulfate reagents. Anal Chem. 1970;42(12):1423-7. [Link] [DOI:10.1021/ac60294a044]
14. Rice EW. Triglycerides in serum. In Standards Methods in Clinical Chemistry. New York: Academic Press; 1970. [Link]
15. Van Handel E. Suggested modifications of the micro determination of triglycerides. Clin Chem. 1961;7:249-51. [Link] [DOI:10.1093/clinchem/7.3.249]
16. Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polarlipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5(5):494-6. [Link] [DOI:10.1007/BF02531316]
17. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170-5. [Link] [DOI:10.1016/S0021-9258(19)45228-9]
18. Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121-6. [Link] [DOI:10.1016/S0076-6879(84)05016-3]
19. Wendel A, Feuerstein S, Konz K-H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979;28(13):2051-5. [Link] [DOI:10.1016/0006-2952(79)90223-5]
20. Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3-11. [Link] [DOI:10.1016/0076-6879(79)62181-X]
21. Naziroglu M, Karaoglu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicol. 2004;195(2-3):221-30. [Link] [DOI:10.1016/j.tox.2003.10.012]
22. Joan O, Barbara AF, Qing X, Samuel WF. The identification of stem cells in human liver diseases and hepatocellular carcinoma. Exp Mol Pathol. 2010;88(3):331-40. [Link] [DOI:10.1016/j.yexmp.2010.01.003]
23. Basu S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicol. 2003;189(1-2):113-27. [Link] [DOI:10.1016/S0300-483X(03)00157-4]
24. Pathakoti K, Goodla L, Manubolu M, Tencomnao T. Metabolic alterations and the protective effect of punicalagin against glutamate-induced oxidative toxicity in HT22 cells. Neurotox Res. 2017;31(4):521-31. [Link] [DOI:10.1007/s12640-016-9697-2]
25. Koolen HHF, da Silva FMA, Gozzo FbC, de Souza AQL, de Souza ADL. Antioxidant, antimicrobial activities and characterization of phenolic compounds fromburiti (Mauritia flexuosa L. f.) by UPLC-ESI-MS/MS. Food Res Int. 2013;51(2):467-73. [Link] [DOI:10.1016/j.foodres.2013.01.039]
26. Lamb RG, Snyder JW, Coleman JB. New trends in the prevention of hepatocellular death. Modifiers of calcium movement and of membrane phospholipid metabolism. Boca Raton: CRC Press; 1988. [Link]
27. Coleman JB, Condie LW, Lamb RG. The influence of CCl4 biotransformation on the activation of rat liver phospholipase C in vitro. Toxicol Appl Pharmacol. 1988;95(2):200-7. [Link] [DOI:10.1016/0041-008X(88)90156-1]
28. Miesel R, Sanocka D, Kurpisz M, Kroger H. Antiinflammatory effects of NADPH oxidase inhibitors. Inflamm. 1995;19(3):347-62. [Link] [DOI:10.1007/BF01534392]
29. Aquilano K, Baldelli S, Ciriolo MR. Glutathione: New roles in redox signaling for an old antioxidant. Front Pharmacol. 2014;5:196. [Link] [DOI:10.3389/fphar.2014.00196]
30. Winkler BS. Unequivocal evidence in support of the nonenzymatic redox coupling between glutathione/glutathione disulfide and ascorbic acid/dehydroascorbic acid. Biochim Biophys Acta. 1992;1117(3):287-90. [Link] [DOI:10.1016/0304-4165(92)90026-Q]
31. Halliwell B, Gutteridge JM. Free radicals in biology and medicine. Oxford: Oxford University Press; 2007. [Link]









