GMJ Medicine
eISSN : 2626-3041
Volume 4, Issue 1 (2025)
GMJM 2025, 4(1): 19-23 |
Back to browse issues page
History
Received: 2024/08/27 | Accepted: 2025/02/2 | Published: 2025/03/15
Received: 2024/08/27 | Accepted: 2025/02/2 | Published: 2025/03/15
How to cite this article
Davoodi M, Ahmed F, Panizai M, Obeidavi Z. Effect of Nano-Phytosome of Quercetin on Mice Liver Infected with Plasmodium berghei. GMJM 2025; 4 (1) :19-23
URL: http://gmedicine.de/article-2-245-en.html
URL: http://gmedicine.de/article-2-245-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Social Determinants of Health Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
2- Livestock and Dairy Development Department Balochistan, Quetta, Pakistan
3- Lorestan University of Medical Sciences, Khorramabad, Iran
2- Livestock and Dairy Development Department Balochistan, Quetta, Pakistan
3- Lorestan University of Medical Sciences, Khorramabad, Iran
Keywords:
Malaria [MeSH], Pro-inflammatory Cytokines [MeSH], Nano-Phytosomes [MeSH], Quercetin [MeSH], Liver Damages [MeSH]
| Abstract (HTML) (2226 Views)
Full-Text: (860 Views)
Introduction
Malaria is one of most important diseases especially in tropical regions and it is a big challenge for most people in all over world. Plasmodium spp. are main factor for malaria. Organization like WHO has estimated to be 200 million people involved with malaria [1].
The different factors such as parasite growth, proliferation, and ability of host immune responses could influence pathogenesis of malaria [2]. The different symptoms has been reported for patients involved with malaria including ague, anemia and cerebral and kidney disorders and splenomegaly [3].
It has been accepted to use animal model for study of animals due to limitations for nature and interpretation under in vitro conditions. Plasmodium berghei is known to have specific characteristics and red blood cell (RBC) tropisms have been used as experimental models in human model [4]. Liver is site for infection of malaria and is involved in the diseases. Inflammatory parameters can be used as markers assessment of liver damages.
Some synthetic agents have commonly been used for treatment of malaria such as chloroquine, pyrimethamine, and sulfadoxine-pyrimethamine. There is increasing interest for application of natural agents for treatment of diseases and/or as adjuvant therapy. Natural anti-inflammatory agents may protect patients with malaria from inflammation.
In this connection, the Flavonoids are known to have beneficial properties including antioxidant, antimicrobial, anticancer and anti-inflammatory [5].
Quercetin is a one of polyphenolic flavonoids which is found in fruits, vegetables and herbs and is used for health benefits such as antioxidant, anti-inflammatory and antimicrobial activities [6, 7]. It is known to have major limitations in bioavailability and absorption [8-10]. Inefficiency of absorption could be attributed to inappropriate lipid solubility, big size of molecules that cannot be absorbed through passive diffusion in intestine-bloodstream pathway, and destroy of phenol moiety of Quercetin by bacteria in gastric space [11-14]. It is essential to use a noval carrier for Quercetin [15]. Phytosome is one novel delivery system that could improve absorption and bioavailability of phyto constituent [16].
It seems that Quercetin could protect liver from damages and inflammation by its antioxidant and anti-inflammatory properties. This novel study aimed to evaluate the application of Nano-phytosomes of Quercetin (NQ) as main agent and/or adjuvant therapy for treatment of infection induced by Plasmodium berghei in mice model., in cases where the doctor is not sure of complete reduction, radiography is recommended for control.
Also, in these studies following reduction, even in cases with fractures, no significant new fractures
were seen in control radiography [15].
Due to the fact that radiography before and after reduction requires significant waste of time, the patient is exposed to harmful radiation and imposes a significant material burden on patients, the present study was conducted based on these objectives to see if the physician's clinical judgment in determining the presence of anterior shoulder dislocation is so accurate that preoperative radiography can be ruled out in these patients. Positive results, improvements in the process of diagnosis and treatment of these patients.
Materials and Methods
Preparation of NQ
Thin layer hydration procedure was used to prepare the NQ. Quercetin (Sigma Aldrich; Germany) and soybean phosphatidylcholine (Merck; Germany) were firstly dissolved in methanol (Merck; Germany), but cholesterol (Merck; Germany) was dissolved in dichloromethane (Merck; Germany). The resulted mixture was kept in a flask.
To evaporate the mixture, a rotary evaporator was used and all the solvents were kept to produce a thin dry film. The prepared lipid thin layer had been exposed to nitrogen gas flow and maintained in an overnight and in the room temperature before hydration to ensure the complete removal of the organic solvents. The film was hydrated with distilled water in a rotary at 45°C. Several procedures were applied to reduce the phytosomes size such as bath sonication (Model 8852, Cole-Palmer; USA) in 45°C, homogenization (Heidolph; Germany) with 20,000rpm and probe-sonication method (Sonics VCX 750; USA).
Animals and Bacteria
A total of 60 male BALB/c mice, 10-12 weeks of age and initial weight 27±3g, were used. The mice were intra-peritoneally infected by administration of 106 P. berghei-infected RBCs.
Hydroxychloroquine sulfate (Biogen; India) was used as recommended by WHO in a dose of 2mg/kg body weight daily for 4 days. Mice were acclimatized for 7 days and divided into 5 groups:
1) Mice received 0.9% isotonic saline and considered as negative control (NC);
2) Non-treated infected mice that were considered as positive control (PC);
3) Infected mice treated with 2mg/kg body weight of HF for 4 days (HF);
4) Infected mice treated with 10 mg/kg body weight of NQ for 4 days (NQ); and
5) Infected mice treated with 10mg/kg body weight of NQ and 2 mg/kg body weight of HF for 4 days (NQ+HF).
A schematic design of study is presented in Figure 1.
Histological study and pro-inflammatory cytokines
In day 28, following scarification, liver was separated and fixed in 10% neutral buffered formalin. Samples were inserted into paraffin and histological changes were evaluated. Scores were considered in scale of 0 up to 4, including not observed (0), very mild (1), mild (2), moderate (3), and severe (4). Blood samples were collected and tumor necrosis factor (TNF)-α and interleukin (IL)-1β levels in the serum were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits.
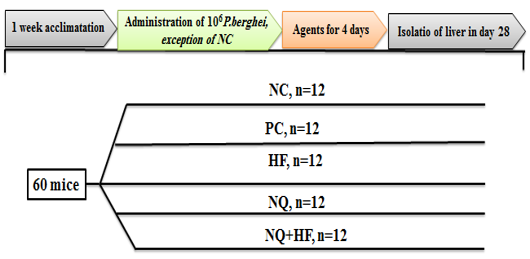
Figure 1. Experimental design and groups
Statistical analyses
The data were reported as mean±standard deviation. One-way ANOVA with Tukey's post hoc tests were applied to compare among groups.
Findings
Histo-pathological parameters
Administration of P. berghei could increase scores for histo-pathological parameters in terms of kupffer cell hyperplasia, hemosiderosis, hepatic necrosis and periportal inflammation in comparison to NC and PC groups (p<0.0001; Figure 2). Administration of HF and NQ could alleviate adverse effects of P. berghei on histo-pathological parameters (p<0.05). The responses were better in HF in comparison to NQ (p<0.05). A combination of NQ and HF could have better effects on histo-pathological parameters in comparison to single form (p<0.05).
Pro-inflammatory cytokines
The data for pro-inflammatory cytokines are shown in Figure 3.
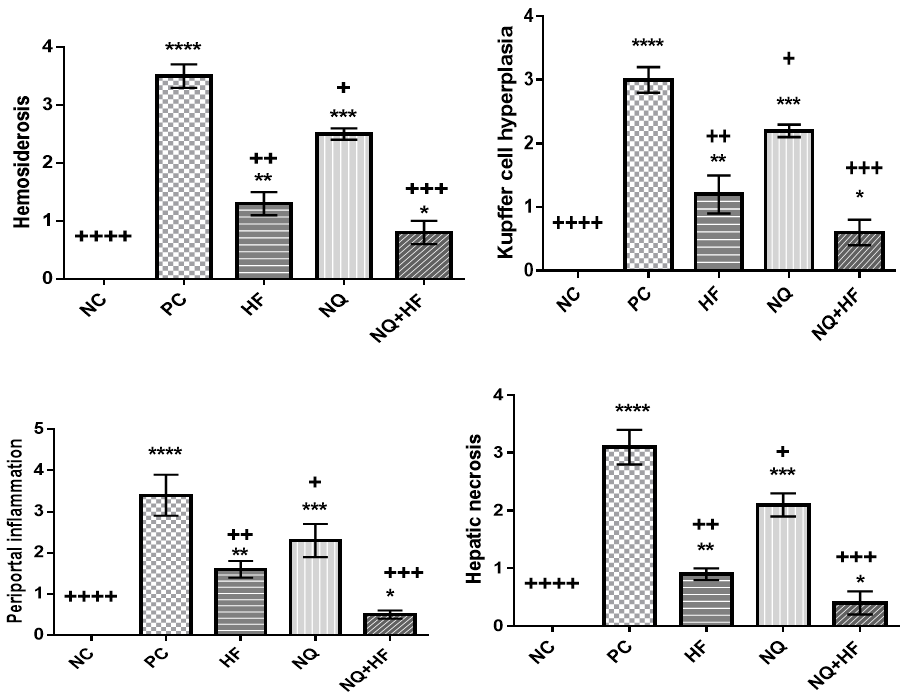
Figure 2. Effects of experimental treatments on histo-pathological parameters of mice involved with malaria (Significant difference between NC group and other groups in 0.05*, 0.01**, 0.001***, and 0.0001****; Significant difference between PC group and other groups in 0.05+, 0.01++, 0.001+++, and 0.0001++++)
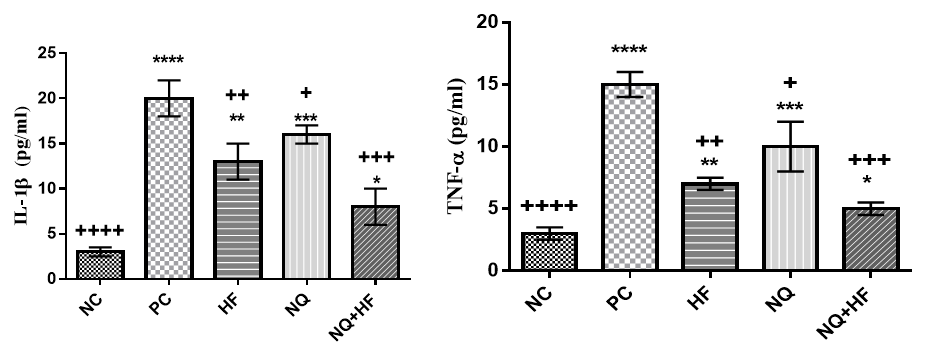
Figure 3. Effects of experimental treatments on serum pro-inflammatory cytokines of mice involved with malaria (Significant difference between NC group and other groups in 0.05*, 0.01**, 0.001***, and 0.0001****; Significant difference between PC group and other groups in 0.05+, 0.01++, 0.001+++, and 0.0001++++)
The levels of inflammatory cytokines were significantly higher in PC group in comparison to NC group (p<0.0001). Administration of NQ, HF and specially their combination could significantly decrease levels of pro-inflammatory cytokines (p<0.05).
Discussion
Malaria is a disease that needs more attentions because it is not still exterminated and the best control procedure is application of a true treatment procedure. Anti-malarial drugs have been used for treatment of malaria but it is essential to investigate their toxicity and damage [17]. The resulted findings for histo-pathological parameters in positive group show that proliferation of the parasites in the blood. As malaria progresses, hepatocytes are destroyed and merozoites are transferred into the blood circulation. Our findings for cytokines confirm such claim. Our findings for kupffer cell hyperplasia showed to be lower in the treated groups and had difference with PC group; showing that the agents could prevent proliferation of liver macrophages. Following the rupture the RBCs, hemoglobin splitting is done and hemosiderosis occurs [18]. In the present study HF and NQ, especially in combination form could alleviate the adverse effects of malaria. The Flavonoids are known to have most common group of polyphenolic compounds and have antioxidant, antimicrobial and anti-inflammatory properties [5].
In summary, NQ showed better activity in combination with HF. NQ has low water solubility and its application in nano-phytosom form could improve solubility and help more absorption. Activated host immune system increases fight against infection, whereas overproduced inflammation causes to produce the tissue damage or even multiple organ failure. It has been shown that the flavonoids and triterpenoids may encourage to treat that could be attributed to antimicrobial properties [19]. Nuclear factor-kappa B (NF-κB) is one mediator for inflammatory response which increases inflammatory cytokines including TNF-α and IL-1β [20]. These findings suggest that NQ does not have effects such as HF, but in combination with HF could increase its efficiency. Future studies are needed to evaluate the effects of NQ on improvement of conditions in mice with malaria, but our findings suggest that NQ can be used as adjuvant therapy in treatment of malaria.
Conclusion
Nano-phytosomes of Quercetin can alleviate adverse effects of malaria in terms of liver damages and inflammation.
Acknowledgements: None declared by the authors.
Ethical Permission: Compliance with ethical guidelines
Approval for this study was obtained from Lorestan University of Medical Sciences Research Committee.
Conflicts of Interests: None declared by the authors.
Authors' Contributions: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Malaria is one of most important diseases especially in tropical regions and it is a big challenge for most people in all over world. Plasmodium spp. are main factor for malaria. Organization like WHO has estimated to be 200 million people involved with malaria [1].
The different factors such as parasite growth, proliferation, and ability of host immune responses could influence pathogenesis of malaria [2]. The different symptoms has been reported for patients involved with malaria including ague, anemia and cerebral and kidney disorders and splenomegaly [3].
It has been accepted to use animal model for study of animals due to limitations for nature and interpretation under in vitro conditions. Plasmodium berghei is known to have specific characteristics and red blood cell (RBC) tropisms have been used as experimental models in human model [4]. Liver is site for infection of malaria and is involved in the diseases. Inflammatory parameters can be used as markers assessment of liver damages.
Some synthetic agents have commonly been used for treatment of malaria such as chloroquine, pyrimethamine, and sulfadoxine-pyrimethamine. There is increasing interest for application of natural agents for treatment of diseases and/or as adjuvant therapy. Natural anti-inflammatory agents may protect patients with malaria from inflammation.
In this connection, the Flavonoids are known to have beneficial properties including antioxidant, antimicrobial, anticancer and anti-inflammatory [5].
Quercetin is a one of polyphenolic flavonoids which is found in fruits, vegetables and herbs and is used for health benefits such as antioxidant, anti-inflammatory and antimicrobial activities [6, 7]. It is known to have major limitations in bioavailability and absorption [8-10]. Inefficiency of absorption could be attributed to inappropriate lipid solubility, big size of molecules that cannot be absorbed through passive diffusion in intestine-bloodstream pathway, and destroy of phenol moiety of Quercetin by bacteria in gastric space [11-14]. It is essential to use a noval carrier for Quercetin [15]. Phytosome is one novel delivery system that could improve absorption and bioavailability of phyto constituent [16].
It seems that Quercetin could protect liver from damages and inflammation by its antioxidant and anti-inflammatory properties. This novel study aimed to evaluate the application of Nano-phytosomes of Quercetin (NQ) as main agent and/or adjuvant therapy for treatment of infection induced by Plasmodium berghei in mice model., in cases where the doctor is not sure of complete reduction, radiography is recommended for control.
Also, in these studies following reduction, even in cases with fractures, no significant new fractures
were seen in control radiography [15].
Due to the fact that radiography before and after reduction requires significant waste of time, the patient is exposed to harmful radiation and imposes a significant material burden on patients, the present study was conducted based on these objectives to see if the physician's clinical judgment in determining the presence of anterior shoulder dislocation is so accurate that preoperative radiography can be ruled out in these patients. Positive results, improvements in the process of diagnosis and treatment of these patients.
Materials and Methods
Preparation of NQ
Thin layer hydration procedure was used to prepare the NQ. Quercetin (Sigma Aldrich; Germany) and soybean phosphatidylcholine (Merck; Germany) were firstly dissolved in methanol (Merck; Germany), but cholesterol (Merck; Germany) was dissolved in dichloromethane (Merck; Germany). The resulted mixture was kept in a flask.
To evaporate the mixture, a rotary evaporator was used and all the solvents were kept to produce a thin dry film. The prepared lipid thin layer had been exposed to nitrogen gas flow and maintained in an overnight and in the room temperature before hydration to ensure the complete removal of the organic solvents. The film was hydrated with distilled water in a rotary at 45°C. Several procedures were applied to reduce the phytosomes size such as bath sonication (Model 8852, Cole-Palmer; USA) in 45°C, homogenization (Heidolph; Germany) with 20,000rpm and probe-sonication method (Sonics VCX 750; USA).
Animals and Bacteria
A total of 60 male BALB/c mice, 10-12 weeks of age and initial weight 27±3g, were used. The mice were intra-peritoneally infected by administration of 106 P. berghei-infected RBCs.
Hydroxychloroquine sulfate (Biogen; India) was used as recommended by WHO in a dose of 2mg/kg body weight daily for 4 days. Mice were acclimatized for 7 days and divided into 5 groups:
1) Mice received 0.9% isotonic saline and considered as negative control (NC);
2) Non-treated infected mice that were considered as positive control (PC);
3) Infected mice treated with 2mg/kg body weight of HF for 4 days (HF);
4) Infected mice treated with 10 mg/kg body weight of NQ for 4 days (NQ); and
5) Infected mice treated with 10mg/kg body weight of NQ and 2 mg/kg body weight of HF for 4 days (NQ+HF).
A schematic design of study is presented in Figure 1.
Histological study and pro-inflammatory cytokines
In day 28, following scarification, liver was separated and fixed in 10% neutral buffered formalin. Samples were inserted into paraffin and histological changes were evaluated. Scores were considered in scale of 0 up to 4, including not observed (0), very mild (1), mild (2), moderate (3), and severe (4). Blood samples were collected and tumor necrosis factor (TNF)-α and interleukin (IL)-1β levels in the serum were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits.

Figure 1. Experimental design and groups
Statistical analyses
The data were reported as mean±standard deviation. One-way ANOVA with Tukey's post hoc tests were applied to compare among groups.
Findings
Histo-pathological parameters
Administration of P. berghei could increase scores for histo-pathological parameters in terms of kupffer cell hyperplasia, hemosiderosis, hepatic necrosis and periportal inflammation in comparison to NC and PC groups (p<0.0001; Figure 2). Administration of HF and NQ could alleviate adverse effects of P. berghei on histo-pathological parameters (p<0.05). The responses were better in HF in comparison to NQ (p<0.05). A combination of NQ and HF could have better effects on histo-pathological parameters in comparison to single form (p<0.05).
Pro-inflammatory cytokines
The data for pro-inflammatory cytokines are shown in Figure 3.

Figure 2. Effects of experimental treatments on histo-pathological parameters of mice involved with malaria (Significant difference between NC group and other groups in 0.05*, 0.01**, 0.001***, and 0.0001****; Significant difference between PC group and other groups in 0.05+, 0.01++, 0.001+++, and 0.0001++++)

Figure 3. Effects of experimental treatments on serum pro-inflammatory cytokines of mice involved with malaria (Significant difference between NC group and other groups in 0.05*, 0.01**, 0.001***, and 0.0001****; Significant difference between PC group and other groups in 0.05+, 0.01++, 0.001+++, and 0.0001++++)
The levels of inflammatory cytokines were significantly higher in PC group in comparison to NC group (p<0.0001). Administration of NQ, HF and specially their combination could significantly decrease levels of pro-inflammatory cytokines (p<0.05).
Discussion
Malaria is a disease that needs more attentions because it is not still exterminated and the best control procedure is application of a true treatment procedure. Anti-malarial drugs have been used for treatment of malaria but it is essential to investigate their toxicity and damage [17]. The resulted findings for histo-pathological parameters in positive group show that proliferation of the parasites in the blood. As malaria progresses, hepatocytes are destroyed and merozoites are transferred into the blood circulation. Our findings for cytokines confirm such claim. Our findings for kupffer cell hyperplasia showed to be lower in the treated groups and had difference with PC group; showing that the agents could prevent proliferation of liver macrophages. Following the rupture the RBCs, hemoglobin splitting is done and hemosiderosis occurs [18]. In the present study HF and NQ, especially in combination form could alleviate the adverse effects of malaria. The Flavonoids are known to have most common group of polyphenolic compounds and have antioxidant, antimicrobial and anti-inflammatory properties [5].
In summary, NQ showed better activity in combination with HF. NQ has low water solubility and its application in nano-phytosom form could improve solubility and help more absorption. Activated host immune system increases fight against infection, whereas overproduced inflammation causes to produce the tissue damage or even multiple organ failure. It has been shown that the flavonoids and triterpenoids may encourage to treat that could be attributed to antimicrobial properties [19]. Nuclear factor-kappa B (NF-κB) is one mediator for inflammatory response which increases inflammatory cytokines including TNF-α and IL-1β [20]. These findings suggest that NQ does not have effects such as HF, but in combination with HF could increase its efficiency. Future studies are needed to evaluate the effects of NQ on improvement of conditions in mice with malaria, but our findings suggest that NQ can be used as adjuvant therapy in treatment of malaria.
Conclusion
Nano-phytosomes of Quercetin can alleviate adverse effects of malaria in terms of liver damages and inflammation.
Acknowledgements: None declared by the authors.
Ethical Permission: Compliance with ethical guidelines
Approval for this study was obtained from Lorestan University of Medical Sciences Research Committee.
Conflicts of Interests: None declared by the authors.
Authors' Contributions: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
1. World Health Organization. Antimicrobial resistance global report on surveillance: 2014 Summary [Internet]. Geneva: World Health Organization; 2014 [cited 2022 November 20]. Available from: https://apps.who.int/iris/bitstream/handle/10665/112647/WHO_HSE_PED_AIP_2014.2_eng.pdf;jsessionid=CADCEAFC2D8E52E06F32874A18A28CBE?sequence=1. [Link]
2. Reisinger EC, Fritzsche C, Krause R, Krejs GJ. Diarrhea caused by primarily non- gastrointestinal infections. Nat Clin Pract Gastroenterol Hepatol. 2005;2(5):216-22. [Link] [DOI:10.1038/ncpgasthep0167]
3. Vafaei MR, Kalani H, Pestehchian N, Nematolahi P. Histopathologic study on mice infected with Plasmodium berghei after treatment with sulfadoxine- pyrimethamine, pyrimethamine, and hydroxychloroquine sulfate. Compar Clin Pathol. 2017;27:123-9. [Link] [DOI:10.1007/s00580-017-2563-7]
4. Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5(9):722-35. [Link] [DOI:10.1038/nri1686]
5. Mahajan TMC, Chaudhari G. A novel approach towards phytosomal flavonoids. Pharm Sci. 2012;4:2079-121. [Link] [DOI:10.4103/0975-7406.94144]
6. Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32(7):1141-8. [Link] [DOI:10.1016/0006-2952(83)90262-9]
7. Hollman PCH, Trijp JMP, Buysman M, Gaag M, Mengelers M, Vries J, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418(1-2):152-6. [Link] [DOI:10.1016/S0014-5793(97)01367-7]
8. Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti- inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56(9):683-7. [Link] [DOI:10.1016/S0014-827X(01)01111-9]
9. Vijayalakshmi A, Ravichandiran V, Velraj M, Nirmala S, Jayakumari S. Screening of flavonoid "quercetin" from the rhizome of Smilax china Linn. for anti- psoriatic activity. Asian Pac J Trop Biomed. 2012;2(4):269-75. [Link] [DOI:10.1016/S2221-1691(12)60021-5]
10. Wang L, Wang B, Li H, Lu H, Qiu F, Xiong L, et al. Quercetin, a flavonoid with anti-inflammatory activity, suppresses the development of abdominal aortic aneurysms in mice. Eur J Pharmacol. 2012;690(1-3):133-41. [Link] [DOI:10.1016/j.ejphar.2012.06.018]
11. Bose S, Michniak Kohn B. Preparation and characterization of lipid based nanosystems for topical delivery of quercetin. Eur J Pharm Sci. 2013;48(3):442-52. [Link] [DOI:10.1016/j.ejps.2012.12.005]
12. Li H, Zhao X, Ma Y, Zhai G, Li L, Lou H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J Control Release. 2009;133(3):238-44. [Link] [DOI:10.1016/j.jconrel.2008.10.002]
13. Liu D, Hu H, Lin Z, Chen D, Zhu Y, Hou S, et al. Quercetin deformable liposome: Preparation and efficacy against ultraviolet B induced skin damages in vitro and in vivo. J Photochem Photobiol B. 2013;127:8-17. [Link] [DOI:10.1016/j.jphotobiol.2013.07.014]
14. Xie Y, Luo H, Duan J, Hong C, Ma P, Li G, et al. Phytic acid enhances the oral absorption of isorhamnetin, quercetin, and kaempferol in total flavones of Hippophae rhamnoides L. Fitoterapia. 2014;93:216-25. [Link] [DOI:10.1016/j.fitote.2014.01.013]
15. Yang B, Chen F, Hua Y, Huang SS, Lin S, Wen L, et al. Prooxidant activities of quercetin, p- courmaric acid and their derivatives analysed by quantitative structure- activity relationship. Food Chem. 2012;131(3):508-12. [Link] [DOI:10.1016/j.foodchem.2011.09.014]
16. Saonere Suryawanshi JA. Phytosomes: An emerging trend in herbal drug treatment. J Med Genet Genomic. 2011;3(6):109-14. [Link]
17. Sharma L, Shukla G. Treatment of pregnant BALB/c mice with sulphadoxine pyrimethamine or chloroquine abrogates Plasmodium berghei induced placental pathology. Parasitol Int. 2014;63(1):49-56. [Link] [DOI:10.1016/j.parint.2013.08.016]
18. Satoskar AR, Simon GL, Hotez PJ, Tsuji M. Medical parasitology Austin. Florida: CRC Press; 2009. [Link] [DOI:10.1201/9781498713672]
19. Badri PN, Renu S. Role of medicinal plants in wound healing. Res J Med Plant. 2011;5(4):392-405. [Link] [DOI:10.3923/rjmp.2011.392.405]
20. Eo H, Lee HJ, Lim Y. Ameliorative effect of dietary genistein on diabetes induced hyper-inflammation and oxidative stress during early stage of wound healing in alloxan induced diabetic mice. Biochem Biophys Res Commun. 2016;478:1021-7. [Link] [DOI:10.1016/j.bbrc.2016.07.039]









