GMJ Medicine
eISSN : 2626-3041
Volume 4, Issue 2 (2025)
GMJM 2025, 4(2): 47-51 |
Back to browse issues page
History
Received: 2024/10/29 | Accepted: 2025/03/16 | Published: 2025/04/20
Received: 2024/10/29 | Accepted: 2025/03/16 | Published: 2025/04/20
How to cite this article
Mesbahzadeh B, Garmsiri M, Jalalvand F, Shojaie L, Kakar M. Effect of Oral Menthol on Antioxidant Status of Polycystic Ovarian Syndrome-Induced Wister Rats. GMJM 2025; 4 (2) :47-51
URL: http://gmedicine.de/article-2-250-en.html
URL: http://gmedicine.de/article-2-250-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
1- Cardiovascular Diseases Research Center, Birjand University of Medical Sciences, Birjand, Iran
2- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran
3- Department of General Surgery, Lorestan University of Medical Sciences, Khorramabad, Iran
4- Tehran University of Medical Sciences, Tehran, Iran
5- L&DD Department, Spinny Road Quetta, Balochistan, Pakistan
2- Student Research Committee, Lorestan University of Medical Sciences, Khorramabad, Iran
3- Department of General Surgery, Lorestan University of Medical Sciences, Khorramabad, Iran
4- Tehran University of Medical Sciences, Tehran, Iran
5- L&DD Department, Spinny Road Quetta, Balochistan, Pakistan
Keywords:
Glucose [MeSH], Lipid Profile [MeSH], Oxidation [MeSH], Polycystic Ovarian Syndrome [MeSH], Rats [MeSH]
| Abstract (HTML) (2676 Views)
Full-Text: (1174 Views)
Introduction
Polycystic ovarian syndrome (PCOS), so called Stein-Leventhal syndrome has been known to have metabolic and reproductive endocrinopathy disorders [1]. Prevalence of PCOS has been reported to be 5-20% in reproductive age [2]. Patients with PCOS are highly sensitive to some diseases and disorders including obesity, insulin resistance, type II diabetes, cardiovascular disease, infertility, malignancy, and psychological disorders [2].
The exact etiology of the PCOS is still unknown, but could be attributed to complex interactions among different factors including genetic, environmental, and behavioral factors. Anxiety, depression, and poor quality of life have been diagnosed in patients with PCOS [3]. Insulin has been known as one of the atherogenic hormones [4] which along with hyperinsulinemia could participate in the development of diabetes, hypertension, and dyslipidemia, which is often combined with elevated total cholesterol and low-density lipoprotein (LDL), triglyceride (TG), and reduced high-density lipoprotein (HDL) levels in patients with PCOS [5]. Hyperinsulinemia has been known to have the ability to promote ovarian androgen overproduction [6]. Dyslipidemia and sex steroids have also been known to have important effects on cardiovascular diseases [7]. Elevated oxidative stress and reduced antioxidant capacity can contribute to the increasing risk of cardiovascular disease in patients with PCOS, in addition to insulin resistance, hypertension, central obesity, and dyslipidemia [7].
The allopathic drugs have commonly been used to treat the PCOS, which include clomiphene citrate, metformin, letrozole, tamoxifene and troglitazone. These drugs have been known to have severe side effects including hot flushes, arthritis, joint or muscle pain and psychological side effects [8]. Since conventional medicine can have side effects, the alternative drugs such as herbal medicines and their derivations can play a significant role. Menthol is one natural cyclic monoterpene alcohol which is found in Mentha species. Menthol is also one of the most important constituents of some essential oils including eucalyptus, lemongrass, and palmarosa [9]. Rozza et al. [10] have shown that menthol can have a gastro protective role against ethanol-induced gastric ulcers and treatment with elevated levels of the anti-inflammatory cytokine IL-10. Menthol has also been known to have antioxidant properties [11].
It seems that menthol has improved antioxidant and lipid profile in animals with PCOS. This study was therefore conducted to evaluate the effects of oral administration of menthol on blood biochemical parameters and antioxidant in mice with PCOS.
Materials and Methods
Preparation of menthol
Menthol oil (Barij Essence; Iran) was crystallized
through chilling in the +14, +10 and –5°C for 8 hours each, respectively, by using sealed plastic containers in freezers. Menthol crystals were isolated from peppermint essential oil because dementholized peppermint essential oil can still contain certain amounts of menthol, racemic and isomenthols and menthone. In order to recover the menthol crystals and to remove the menthone, it had to be treated with 8g boric acid in distillation flask for a period of 3 hours. The rest of the distillation containing borates of menthol were saponified using steam distillation on 70g of 15% NaOH solution, and finally crystals were separated and dried in 26°C and production was investigated.
Animals
Fifty female Wistar rats, 13-15 weeks of age, weighing 170±20g, were purchased from Pasture Institute, Tehran-Iran. To adapt, the female rats were grouped into 5 groups in the controlled temperature of 22-24°C and a lighting diet of 12h light:12h darkness cycle. Food and water were ad libitum supplied.
Induction of PCOS and treatments
The Wistar rats were distributed into five groups:
1) Control group (Control) or healthy rats that did not receive any menthol;
2) PCOS group (PCOS) that did not receive any menthol;
3) PCOS induced rats that received 2mg/kg of body weight of menthol (PCOS-2)
4) PCOS induced rats that received 4mg/kg of body weight of menthol (PCOS-4)
5) PCOS induced rats that received 6mg/kg of body weight of menthol (PCOS-6).
In the PCOS induction phase, rats were induced with PCOS by applying 5mg estradiol valerate, as reported by previous studies [12]. Wistar rats were treated with menthol for 28 days. Body weight was recorded in days 1, 14 and 28 post-induction.
Blood sampling and biochemical analyses
At the end of the trial, all the Wistar rats were anesthetized by using ketamine/xylazine HCl (75/10mg/kg intraperitoneally). The collected blood samples from the aorta with anticoagulant were centrifuged for 12min in 3500rpm/min to achieve the plasma and stored in -20°C until biochemical analysis for investigation of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C. The plasma concentrations of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C were investigated by using Pars Azmoon commercial Kits.
Ferric-reducing ability of plasma (FRAP)
FRAP reagent was produced and heated in 37°C while also a mixture of the following solutions was applied [14]: 1) 0.3M sodium acetate buffer solution (pH 3.6), 2) 10mM 2,4,6-tripyridyl-1-5-triazine in 40mM HCl solution, and 3) 20 mM FeCl3 solution at the ratio of 10:1:1 (v/v/v). A level of plasma (10μL) was incubated along along with 90μL of FRAP reagent in a micro plate for 30 minutes in room temperature in the dark. The level of absorbance of the mixture was assessed in the wavelength of 595nm by a spectrophotometer. The levels of FRAP values were measured by a calibration standard curve of FeSO4 (0-2000μM).
Advanced oxidation protein product (AOPP)
Samples were made as follows: in a tube, 20μL of plasma from each rat was diluted into 100μL in phosphate-buffered saline by the inclusion of 10μL of 1.16M KI and 20μL of absolute acetic acid. The absorbance of the reaction mixture was rapidly read by a SpectraMax1601 spectrophotometer (Sunnyvale; USA) in 340nm against a blank, having 100μL of phosphate-buffered saline, 20μL of acetic acid, and 10μL of KI solution. As the linear range of chloramine-T absorbance in 340nm is between 0 and 100μM, AOPP concentrations were reported in μM chloramine-T equivalents. All evaluations were conducted simultaneously.
Total oxidation status (TOS)
The plasma concentration of TOS was evaluated by a colorimetric measurement procedure. In order to evaluate the TOS, 225μL of Reagent 1 (xylenol orange 150μM, NaCl 140mM, and glycerol 1.35M in 25 mMH2SO4 solution, pH 1.75) was mixed with 35μL of plasma sample, and the absorbance of each sample was investigated spectrophotometrically in 560nm as a sample blank. Then, 11μL of Reagent 2 (ferrous ion (5mM) and o-dianisidine (10mM) in 25mM H2SO4 solution) was added to the mixture within 3-4 min. The last absorbance was investigated in 560nm. The assay was calibrated by using H2O2, and the results have been reported in terms of micromolar H2O2 equivalent per liter (μmol H2O2equiv/L). The detection limit of the procedure was investigated by evaluating the zero calibrator 10 times.
Statistical analysis
The statistical analyses were conducted via GraphPad Prism software. The analysis of variance (ANOVA) was used to compare among the groups, and post hoc (Tukey) was used to compare the groups. Significance was considered in p<0.05.
Findings
Body weight
Effects of different levels of menthol on body weight of Wistar rats are presented in Figure 1. As the results indicate, body weight was not influenced by experimental treatments in day 1 (p>0.05). There was no significant difference between control and PCOS or control PCOS in day 1 (p>0.05). In days 14 and 28, induction of PCOS significantly increased body weight, so control PCOS rats showed higher body weight in comparison to control group (p<0.05). Oral gavage of menthol could significantly decrease body weight. There was positive correlation between body weight and level of PCOS (p<0.05), so higher levels of menthol could significantly decrease body weight (p<0.05).
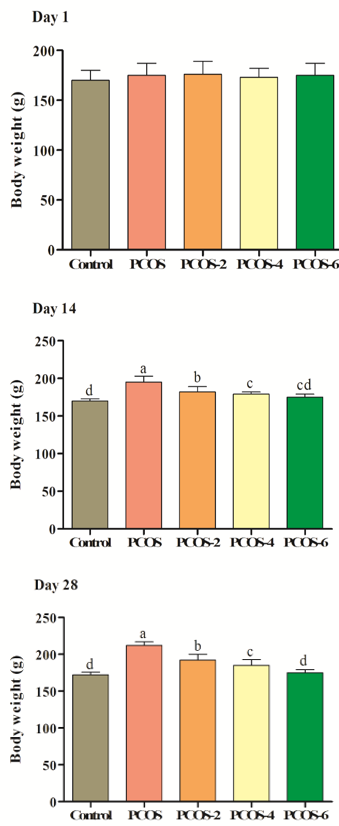
Figure 1. Effects of different levels of menthol (2, 4 & 6mg/kg) on body weight (g) in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6 mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Blood biochemical parameters
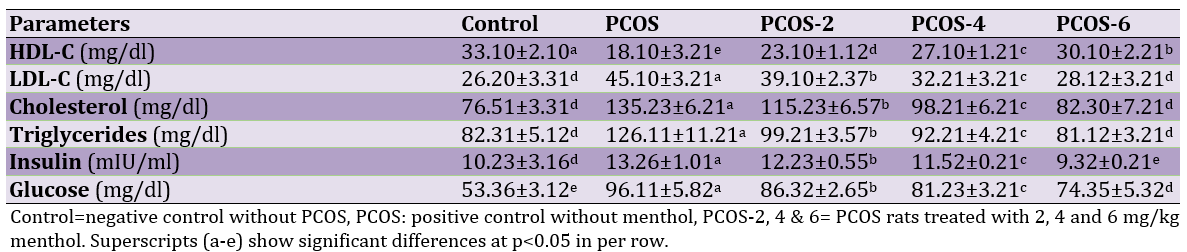
Induction of PCOS could significantly increase LDL-C, cholesterol, triglycerides, insulin and glucose but also decrease HDL-C (control versus PCOS group (p<0.05). However, oral administration of menthol could significantly increase HDL-C and decrease LDL-C, cholesterol, triglycerides, insulin and glucose (p<0.05). The best responses were observed in highest levels of menthol (6mg/kg; Table 1).
Table 1. Effects of different levels of menthol on blood biochemical parameters in rats with PCOS
Antioxidant status
The induction of PCOS increased oxidation in terms of FRAP, AOPP and TOS (p<0.05; control vs. PCOS). Oral treatment with menthol significantly improved antioxidant levels in comparison to PCOS group. As levels of menthol were increased, oxidation status was decreased respectively (Figure 2).
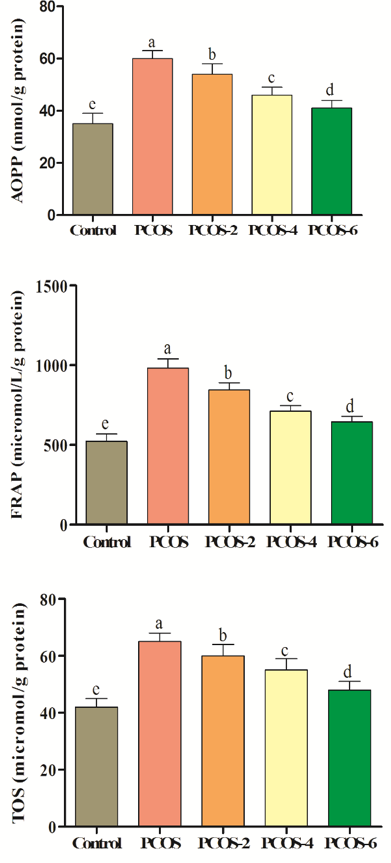
Figure 2. Effects of different levels of menthol (2, 4 & 6mg/kg) on antioxidant status in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Discussion
Body weight significantly increased in PCOS rats compared to the control group. Kim et al. [14] showed in their study that increased body weight in PCOS rats induced with dehydroepiandrosterone. The difference between control group and PCOS group could be attributed to inferring obesity [15], and diets can have a major role in weight gain in animals with PCOS. It has been accepted that high-fiber diet and low-fat diets could decrease body weight [16]. In the current study, menthol comprises very small portion of the diet and thus cannot have a role due to low fat levels. It seems that menthol decreases oxidation and subsequent dyslipidemia, and dyslipidemia could have a major role in increased body weight. Maintained body weight could be attributed to antioxidant status.
Hyperglycemia was observed in rats with PCOS. PCOS has been reported as one metabolic disorder related with type 2 diabetes mellitus [17] and it is caused by hyperglycemia in initial phases which leads to insulin resistance. Similarly, other studies have reported hyperglycemia in Letrozole induced PCOS rats [18]. Hyperinsulinemia was also observed in our study and it is known to have the ability to promote ovarian androgen overproduction [6]. Menthol decreased levels of insulin and glucose, and it seemed that menthol increases sensitivity to insulin and decreases glucose.
Dyslipidemia was also observed in rats with PCOS (control vs. PCOS). Imbalanced lipid profile has been related to hyper-androgenemia [19, 20]. Lipid peroxidation has been known as one of the markers for oxidative tissue damage. It also induces free radical damage to the components of cell membrane that cause cell necrosis and inflammation [21]. Some studies have reported oxidative stress as one of the pathological factors for PCOS [22, 23].
Elevated oxidant levels could change the stereo diagnosis in ovaries which could be attributed to raised androgen production and polycystic ovaries [22]. In the present study, menthol improves lipid profile by antioxidant system, and our findings for oxidation system supported our hypotheses. In sum, it could be stated that antioxidant activity of menthol prevents lipid peroxidation and blood parameters could be considered as index for such action. Future studies are needed to evaluate the effects of menthol. Therefore, we recommend the use of menthol for treatment of PCOS as a novel agent.
Conclusion
PCOS has negative effects on almost all blood parameters as well as the antioxidant status. Oral administration of menthol improves blood parameters through antioxidant system.
Acknowledgements: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: None declared by the authors.
Polycystic ovarian syndrome (PCOS), so called Stein-Leventhal syndrome has been known to have metabolic and reproductive endocrinopathy disorders [1]. Prevalence of PCOS has been reported to be 5-20% in reproductive age [2]. Patients with PCOS are highly sensitive to some diseases and disorders including obesity, insulin resistance, type II diabetes, cardiovascular disease, infertility, malignancy, and psychological disorders [2].
The exact etiology of the PCOS is still unknown, but could be attributed to complex interactions among different factors including genetic, environmental, and behavioral factors. Anxiety, depression, and poor quality of life have been diagnosed in patients with PCOS [3]. Insulin has been known as one of the atherogenic hormones [4] which along with hyperinsulinemia could participate in the development of diabetes, hypertension, and dyslipidemia, which is often combined with elevated total cholesterol and low-density lipoprotein (LDL), triglyceride (TG), and reduced high-density lipoprotein (HDL) levels in patients with PCOS [5]. Hyperinsulinemia has been known to have the ability to promote ovarian androgen overproduction [6]. Dyslipidemia and sex steroids have also been known to have important effects on cardiovascular diseases [7]. Elevated oxidative stress and reduced antioxidant capacity can contribute to the increasing risk of cardiovascular disease in patients with PCOS, in addition to insulin resistance, hypertension, central obesity, and dyslipidemia [7].
The allopathic drugs have commonly been used to treat the PCOS, which include clomiphene citrate, metformin, letrozole, tamoxifene and troglitazone. These drugs have been known to have severe side effects including hot flushes, arthritis, joint or muscle pain and psychological side effects [8]. Since conventional medicine can have side effects, the alternative drugs such as herbal medicines and their derivations can play a significant role. Menthol is one natural cyclic monoterpene alcohol which is found in Mentha species. Menthol is also one of the most important constituents of some essential oils including eucalyptus, lemongrass, and palmarosa [9]. Rozza et al. [10] have shown that menthol can have a gastro protective role against ethanol-induced gastric ulcers and treatment with elevated levels of the anti-inflammatory cytokine IL-10. Menthol has also been known to have antioxidant properties [11].
It seems that menthol has improved antioxidant and lipid profile in animals with PCOS. This study was therefore conducted to evaluate the effects of oral administration of menthol on blood biochemical parameters and antioxidant in mice with PCOS.
Materials and Methods
Preparation of menthol
Menthol oil (Barij Essence; Iran) was crystallized
through chilling in the +14, +10 and –5°C for 8 hours each, respectively, by using sealed plastic containers in freezers. Menthol crystals were isolated from peppermint essential oil because dementholized peppermint essential oil can still contain certain amounts of menthol, racemic and isomenthols and menthone. In order to recover the menthol crystals and to remove the menthone, it had to be treated with 8g boric acid in distillation flask for a period of 3 hours. The rest of the distillation containing borates of menthol were saponified using steam distillation on 70g of 15% NaOH solution, and finally crystals were separated and dried in 26°C and production was investigated.
Animals
Fifty female Wistar rats, 13-15 weeks of age, weighing 170±20g, were purchased from Pasture Institute, Tehran-Iran. To adapt, the female rats were grouped into 5 groups in the controlled temperature of 22-24°C and a lighting diet of 12h light:12h darkness cycle. Food and water were ad libitum supplied.
Induction of PCOS and treatments
The Wistar rats were distributed into five groups:
1) Control group (Control) or healthy rats that did not receive any menthol;
2) PCOS group (PCOS) that did not receive any menthol;
3) PCOS induced rats that received 2mg/kg of body weight of menthol (PCOS-2)
4) PCOS induced rats that received 4mg/kg of body weight of menthol (PCOS-4)
5) PCOS induced rats that received 6mg/kg of body weight of menthol (PCOS-6).
In the PCOS induction phase, rats were induced with PCOS by applying 5mg estradiol valerate, as reported by previous studies [12]. Wistar rats were treated with menthol for 28 days. Body weight was recorded in days 1, 14 and 28 post-induction.
Blood sampling and biochemical analyses
At the end of the trial, all the Wistar rats were anesthetized by using ketamine/xylazine HCl (75/10mg/kg intraperitoneally). The collected blood samples from the aorta with anticoagulant were centrifuged for 12min in 3500rpm/min to achieve the plasma and stored in -20°C until biochemical analysis for investigation of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C. The plasma concentrations of glucose, insulin, total cholesterol, triglycerides, HDL-C and LDL-C were investigated by using Pars Azmoon commercial Kits.
Ferric-reducing ability of plasma (FRAP)
FRAP reagent was produced and heated in 37°C while also a mixture of the following solutions was applied [14]: 1) 0.3M sodium acetate buffer solution (pH 3.6), 2) 10mM 2,4,6-tripyridyl-1-5-triazine in 40mM HCl solution, and 3) 20 mM FeCl3 solution at the ratio of 10:1:1 (v/v/v). A level of plasma (10μL) was incubated along along with 90μL of FRAP reagent in a micro plate for 30 minutes in room temperature in the dark. The level of absorbance of the mixture was assessed in the wavelength of 595nm by a spectrophotometer. The levels of FRAP values were measured by a calibration standard curve of FeSO4 (0-2000μM).
Advanced oxidation protein product (AOPP)
Samples were made as follows: in a tube, 20μL of plasma from each rat was diluted into 100μL in phosphate-buffered saline by the inclusion of 10μL of 1.16M KI and 20μL of absolute acetic acid. The absorbance of the reaction mixture was rapidly read by a SpectraMax1601 spectrophotometer (Sunnyvale; USA) in 340nm against a blank, having 100μL of phosphate-buffered saline, 20μL of acetic acid, and 10μL of KI solution. As the linear range of chloramine-T absorbance in 340nm is between 0 and 100μM, AOPP concentrations were reported in μM chloramine-T equivalents. All evaluations were conducted simultaneously.
Total oxidation status (TOS)
The plasma concentration of TOS was evaluated by a colorimetric measurement procedure. In order to evaluate the TOS, 225μL of Reagent 1 (xylenol orange 150μM, NaCl 140mM, and glycerol 1.35M in 25 mMH2SO4 solution, pH 1.75) was mixed with 35μL of plasma sample, and the absorbance of each sample was investigated spectrophotometrically in 560nm as a sample blank. Then, 11μL of Reagent 2 (ferrous ion (5mM) and o-dianisidine (10mM) in 25mM H2SO4 solution) was added to the mixture within 3-4 min. The last absorbance was investigated in 560nm. The assay was calibrated by using H2O2, and the results have been reported in terms of micromolar H2O2 equivalent per liter (μmol H2O2equiv/L). The detection limit of the procedure was investigated by evaluating the zero calibrator 10 times.
Statistical analysis
The statistical analyses were conducted via GraphPad Prism software. The analysis of variance (ANOVA) was used to compare among the groups, and post hoc (Tukey) was used to compare the groups. Significance was considered in p<0.05.
Findings
Body weight
Effects of different levels of menthol on body weight of Wistar rats are presented in Figure 1. As the results indicate, body weight was not influenced by experimental treatments in day 1 (p>0.05). There was no significant difference between control and PCOS or control PCOS in day 1 (p>0.05). In days 14 and 28, induction of PCOS significantly increased body weight, so control PCOS rats showed higher body weight in comparison to control group (p<0.05). Oral gavage of menthol could significantly decrease body weight. There was positive correlation between body weight and level of PCOS (p<0.05), so higher levels of menthol could significantly decrease body weight (p<0.05).

Figure 1. Effects of different levels of menthol (2, 4 & 6mg/kg) on body weight (g) in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6 mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Blood biochemical parameters
Induction of PCOS could significantly increase LDL-C, cholesterol, triglycerides, insulin and glucose but also decrease HDL-C (control versus PCOS group (p<0.05). However, oral administration of menthol could significantly increase HDL-C and decrease LDL-C, cholesterol, triglycerides, insulin and glucose (p<0.05). The best responses were observed in highest levels of menthol (6mg/kg; Table 1).
Table 1. Effects of different levels of menthol on blood biochemical parameters in rats with PCOS

Antioxidant status
The induction of PCOS increased oxidation in terms of FRAP, AOPP and TOS (p<0.05; control vs. PCOS). Oral treatment with menthol significantly improved antioxidant levels in comparison to PCOS group. As levels of menthol were increased, oxidation status was decreased respectively (Figure 2).

Figure 2. Effects of different levels of menthol (2, 4 & 6mg/kg) on antioxidant status in rats with PCOS. Control=control standard without PCOS, PCOS= PCOS control without menthol, PCOS-2, 4 & 6= PCOS rats treated with 2, 4 and 6mg/kg menthol. Superscripts (a-e) show significant differences at p<0.05.
Discussion
Body weight significantly increased in PCOS rats compared to the control group. Kim et al. [14] showed in their study that increased body weight in PCOS rats induced with dehydroepiandrosterone. The difference between control group and PCOS group could be attributed to inferring obesity [15], and diets can have a major role in weight gain in animals with PCOS. It has been accepted that high-fiber diet and low-fat diets could decrease body weight [16]. In the current study, menthol comprises very small portion of the diet and thus cannot have a role due to low fat levels. It seems that menthol decreases oxidation and subsequent dyslipidemia, and dyslipidemia could have a major role in increased body weight. Maintained body weight could be attributed to antioxidant status.
Hyperglycemia was observed in rats with PCOS. PCOS has been reported as one metabolic disorder related with type 2 diabetes mellitus [17] and it is caused by hyperglycemia in initial phases which leads to insulin resistance. Similarly, other studies have reported hyperglycemia in Letrozole induced PCOS rats [18]. Hyperinsulinemia was also observed in our study and it is known to have the ability to promote ovarian androgen overproduction [6]. Menthol decreased levels of insulin and glucose, and it seemed that menthol increases sensitivity to insulin and decreases glucose.
Dyslipidemia was also observed in rats with PCOS (control vs. PCOS). Imbalanced lipid profile has been related to hyper-androgenemia [19, 20]. Lipid peroxidation has been known as one of the markers for oxidative tissue damage. It also induces free radical damage to the components of cell membrane that cause cell necrosis and inflammation [21]. Some studies have reported oxidative stress as one of the pathological factors for PCOS [22, 23].
Elevated oxidant levels could change the stereo diagnosis in ovaries which could be attributed to raised androgen production and polycystic ovaries [22]. In the present study, menthol improves lipid profile by antioxidant system, and our findings for oxidation system supported our hypotheses. In sum, it could be stated that antioxidant activity of menthol prevents lipid peroxidation and blood parameters could be considered as index for such action. Future studies are needed to evaluate the effects of menthol. Therefore, we recommend the use of menthol for treatment of PCOS as a novel agent.
Conclusion
PCOS has negative effects on almost all blood parameters as well as the antioxidant status. Oral administration of menthol improves blood parameters through antioxidant system.
Acknowledgements: None declared by the authors.
Ethical Permissions: None declared by the authors.
Conflicts of Interests: None declared by the authors.
Authors’ Contribution: All authors contributed toward data analysis, drafting and revising the paper and agreed to be responsible for all the aspects of this work.
Funding/Support: None declared by the authors.
References
1. Patil S, Ramesh S, Murthy Kharidhi HS. A prospective analysis of polycystic ovarian syndrome in infertile women. Int J Reprod Contraception Obstetric Gynecol. 2019;8(1). [Link] [DOI:10.18203/2320-1770.ijrcog20185443]
2. El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: An updated overview. Front Physiol. 2016;7:124. [Link] [DOI:10.3389/fphys.2016.00124]
3. Kolivand M, Keramat A, Khosravi A. The effect of herbal teas of management of polycystic ovary syndrome: A systematic review. J Midwifery Reprod Health. 2017;5(4):1098-106. [Persian] [Link]
4. Wild RA. Cardiovascular disease risks, insulin resistance, and androgen excess. Semin Reprod Endocrinol. 1994;12:38-44. [Link] [DOI:10.1055/s-2007-1016380]
5. Birdsall MA, Farquhar CM, White HD. Association between polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann. Int. Med. 1997;126(1):32-5. [Link] [DOI:10.7326/0003-4819-126-1-199701010-00005]
6. Stuart D, Nagamani M. Acute augmentation of plasma androstenedione and dehydroepiandrosterone by euglycemic insulin infusion: Evidence for a direct effect of insulin on ovarian steroidogenesis. Fertil Steril. 1990;54(5):788-92. [Link] [DOI:10.1016/S0015-0282(16)53931-4]
7. Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil. Steril. 2003;80:123-7. [Link] [DOI:10.1016/S0015-0282(03)00571-5]
8. Sasikala SL, Shamila S. A novel ayurvedic medicine- asokarishtam in the treatment of letrozole induced pcos in rat. J Cell Tissue Res. 9(2):1903-7. [Link]
9. Oz M, El Nebrisi EG, Yang KS, Howarth FC, Al Kury LT. Cellular and molecular targets of menthol actions. Front Pharmacol. 2017;8:472. [Link] [DOI:10.3389/fphar.2017.00472]
10. Rozza AL, Meira de Faria F, Souza Brito AR, Pellizzon CH. The gastro-protective effect of menthol: Involvement of anti-apoptotic, antioxidant andanti-inflammatory activities. PloS. ONE. 2014;9:e86686. [Link] [DOI:10.1371/journal.pone.0086686]
11. Stringaro A, Colone M, Angiolella L. Antioxidant, antifungal, antibiofilm, and cytotoxic activities of Mentha spp. Essential Oils. Medicines (Basel). 2018;5:112-21. [Link] [DOI:10.3390/medicines5040112]
12. Stener Victorin E, Ploj K, Larsson BM, Holmang A. Rats with steroid-induced polycystic ovaries develop hypertension and increased sympathetic nervous system activity. Reprod Biol Endocrinol. 2005;3:44-51. [Link] [DOI:10.1186/1477-7827-3-44]
13. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power: The FRAP assay. Anal Biochem. 1996;239(1):70-6. [Link] [DOI:10.1006/abio.1996.0292]
14. Kim E, Jang M, Choi JH, Park KS, Cho I. An improved dehydroepiandrosterone-induced rat model of polycystic ovary syndrome (PCOS): Post-pubertal improve PCOS's features. Front. Endocrin. 2018;9:735. [Link] [DOI:10.3389/fendo.2018.00735]
15. Cavagni J, Wagner TP, Gaio EJ, Rego RO, Torres IL, et al. Obesity may increase the occurrence of spontaneous periodontal disease in Wistar rats. Arch Oral Biol. 2013;58:1034-9. [Link] [DOI:10.1016/j.archoralbio.2013.03.006]
16. Vignesh JP, Mohan V. Polycystic ovary syndrome: A component of metabolic syndrome?. J Postgrad Med. 2007;53:12-34. [Link] [DOI:10.4103/0022-3859.32217]
17. Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects:results of an 8-year follow-up. Curr Diab Rep. 2006;6(1):77-83. [Link] [DOI:10.1007/s11892-006-0056-1]
18. Radha Maharjan H, Padamnabhi Nagar S, Laxmipriya Nampoothiri P. Effect of Aloe barbadensis Mill. formulation on Letrozole induced polycystic ovarian syndrome rat model. J Ayurveda Integ Med. 2010;1(4):273-9. [Link] [DOI:10.4103/0975-9476.74090]
19. Von Eckardstein A. Androgens, cardiovascular risk factors and atherosclerosis. Testosterone. 1998:229-57. [Link] [DOI:10.1007/978-3-642-72185-4_8]
20. Croston GE, Milan LB, Marschke KB, Reichman M, Briggs MR. Androgen receptor- mediated antagonism of estrogen-dependentlow density lipoprotein receptor transcription in cultured hepatocytes. Endocrinology. 1997;138(9):3779-86. [Link] [DOI:10.1210/endo.138.9.5404]
21. Ayala A, Munoz FM, Arguelles S. Lipid peroxidation: Production, metabolism and signalling mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxidative. Med Cell Longev. 2014;2014:360438. [Link] [DOI:10.1155/2014/360438]
22. Sabuncu T, Vural H, Harma M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin Biochem. 2001;34(5):407-13. [Link] [DOI:10.1016/S0009-9120(01)00245-4]
23. Liu J, Zhang D. The role of oxidative stress in pathogenesis of polycystic ovary syndrome. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43(2):187-90. [Link]









