GMJ Medicine
eISSN : 2626-3041
Volume 4, Issue 2 (2025)
GMJM 2025, 4(2): 71-75 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/09/23 | Accepted: 2025/02/20 | Published: 2025/04/10
Received: 2024/09/23 | Accepted: 2025/02/20 | Published: 2025/04/10
How to cite this article
Rostami Mehr S, Hossein Gholizadeh Salmani R, Abbasi-Maleki S, Rasheed S, Haghipanah M. Beneficial Effects of Exercise on Brain-Derived Neurotrophic Factor in Subjects with Alzheimer; a Systematic Review. GMJM 2025; 4 (2) :71-75
URL: http://gmedicine.de/article-2-262-en.html
URL: http://gmedicine.de/article-2-262-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
S. Rostami Mehr1, R. Hossein Gholizadeh Salmani2, S. Abbasi-Maleki1, S. Rasheed3, M. Haghipanah *4
1- Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
2- Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
3- Department of Medicine Emergency, Acharya Shree Bhikshu Hospital, Moti Nagar, New Delhi, India
4- International Center for Neuroscience Research, Institute for Intelligent Research, Tbilisi, Georgia
2- Department of Basic Sciences, Faculty of Veterinary Medicine, Urmia University, Urmia, Iran
3- Department of Medicine Emergency, Acharya Shree Bhikshu Hospital, Moti Nagar, New Delhi, India
4- International Center for Neuroscience Research, Institute for Intelligent Research, Tbilisi, Georgia
| Abstract (HTML) (1923 Views)
Full-Text: (681 Views)
Introduction
Alzheimer's disease (AD) is a type of brain dysfunction that gradually degrades the patient's mental abilities [1]. It is normal for people to become a little forgetful as they age, but memory disorder progresses gradually in AD [2]. Alzheimer's causes the death of nerve cells and loss of tissue throughout the brain [3]. With the severity of the disease, the brain tissue shrinks, and the areas containing cerebrospinal fluid become larger [4].
The damage caused by Alzheimer's affects the affected person's memory, speech, and perception. Memory problems are usually one of the first symptoms of Alzheimer's [5]. However, the initial symptoms of this disease vary from person to person. Complications such as impaired performance in other aspects of thinking, such as finding the right words while speaking, visual/spatial problems, and impaired reasoning or judgment can be another sign of the early stages of AD [6, 7]. Memory problems are usually one of the first symptoms of Alzheimer's [8]. However, the initial symptoms of this disease vary from person to person. Complications such as impaired performance in other aspects of thinking, such as finding the right words while speaking, visual/spatial problems, and impaired reasoning or judgment can be another sign of the early stages of Alzheimer's disease [9, 10].
Alzheimer's disease affects the secretion of many neurotrophins [11]. These are nerve growth factors and are a type of cytokine. Neurotrophins, including Brain-Derived Neurotrophic Factor (BDNF), are key in increasing sympathetic activity and blood pressure [12, 13]. Studies have shown that the level of BDNF decreases in AD, and the decrease in this factor is consistent with the decrease in hippocampal volume in this disease [14, 15]. BDNF affects the intracellular signaling pathway of the proliferation of hippocampus cells and thus interferes in the development of AD [16, 17].
Alzheimer's has no cure, and there is no way to slow down the rate of nerve damage that it causes in the brain [18]. However, some treatments seem to help maintain mental skills and reduce the effects of the disease. On the other hand, physical exercises can protect against deterioration [19]. Increasing physical activity is effective in different lifestyles and increases brain activity, especially in the hippocampus area (memory center and learning), thus decreasing AD's secondary effects [20, 21]. It seems that the aerobic exercise program can effectively improve the neuropsychological functions of these patients. Several studies have reported the efficiency of exercises on BDNF in AD [22-24], but there is no comprehensive review on the effects of exercise on BDNF in AD. Thus, this study aimed to evaluate the effects of exercises on Brain-Derived Neurotrophic Factor levels in patients with Alzheimer's.
Information and Methods
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25] in 2023. English papers on the effects of exercises on BDNF levels in subjects with Alzheimer's were included. Studies lacking complete data (e.g., lack of subjects, their numbers, periods, sample collection, and unclear results) were excluded. Unpublished papers, review papers, and studies with deficient information were not included. Papers were searched with keywords of “exercise” OR “BDNF” OR “neurotrophins” OR “rats” OR “mice” OR “humans”. The second strategy was to search reference lists in review articles for related articles. Articles were searched based on title and abstract. Duplicate papers were excluded, and potential papers were included. Searches were finalized on September 5th, 2023. Searches lasted for weeks to obtain final papers. Reviews and other mentioned non-related papers were excluded.
A standardized data coding form was prepared to extract the information from each study, including authors and publication year, study sample characteristics (number of subjects and their type), period of study, and results. Each study has only participated in the current research once [26]. In the current study, 12 articles were identified with the help of a database search and searching in a reference list. Keywords were searched in PubMed, Scopus, EuropePMC, Cochrane Central Database, Embase, and Web of Science databases (Figure 1). All the studies were managed with Endnote™ X9.2 (Thomson Reuters, Philadelphia, PA, USA) software. All the papers were read and investigated by two reviewers.
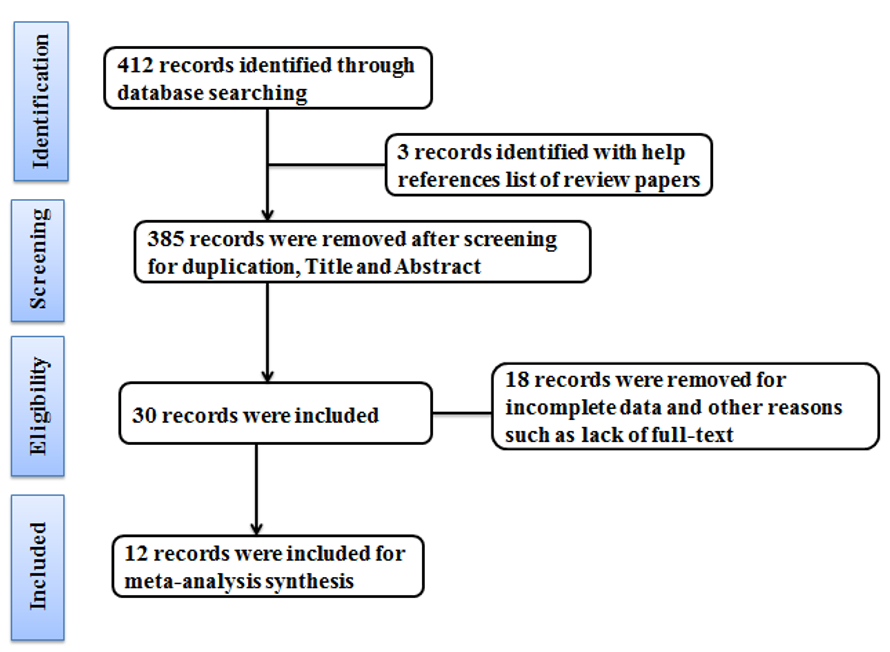
Figure 1. PRISMA flow diagram for included studies for the effects of exercises on BDNF
Findings
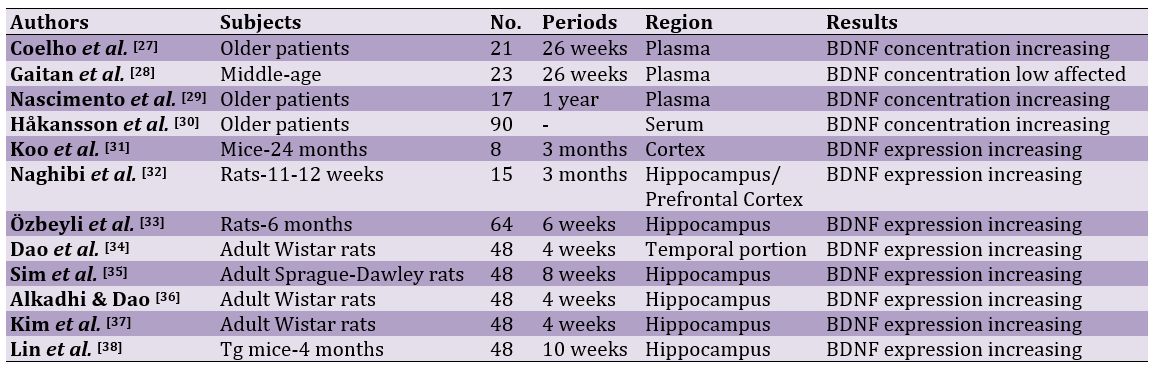
Twelve papers were reviewed, comprising 4 human and 8 animal studies. The periods for human studies lasted 26 to 52 weeks. Animal studies lasted 4 to 13 weeks. The samples were collected from plasma in human studies, while most animal studies were conducted on the expression of BDNF in the hippocampus (n=6). Out of 12 papers, 3 human papers and 8 animal studies showed that exercises significantly increased the concentration of the expression of BDNF (Table 1).
Table 1. Studies conducted on the effects of exercises on BDNF in subjects
Discussion
This systematic review assessed the effects of exercises on BDNF levels in subjects with Alzheimer's. Most human studies were conducted on middle-aged and elderly people. It is a common disease in elderly people. Alzheimer's disease in the elderly is a brain disorder that slowly destroys memory and thinking skills and, ultimately, the ability to do the simplest tasks. Recent estimates show that Alzheimer's disease is the third leading cause of death in the elderly after heart disease and cancer [39, 40].
Alzheimer's in the elderly is the most common cause of dementia among the elderly. Dementia is the loss of cognitive function-thinking, remembering, reasoning, and behavioral abilities to the extent that it interferes with a person's daily life and activities [41]. The severity of dementia ranges from the mildest stage, when it just begins to affect a person's functioning, to the most severe stage, when the person must be completely dependent on others for basic activities of daily living [42]. The damage occurs first in the hippocampus and entorhinal cortex, parts of the brain essential to forming memories. As more nerve cells die, other brain parts are affected and diminish. In the late stage of Alzheimer's in the elderly, the damage is extensive, and the brain tissue has significantly diminished [43].
Most studies on animal models were also conducted on elderly animals. Thus, animal models emphasize aged animals. The studies had differences for periods. Human studies were conducted for longer times, 26 weeks and longer. Since human studies were scarce, a relation between the period and efficiency of exercises can be stated. However, longer and shorter animal studies reported the positive effects. It can be stated that short-term exercises can increase BDNF in animal models. The results also showed that exercises influence the concentration of BDNF in the plasma. A study showed that exercises cannot positively affect the concentration plasma of BDNF [28]. The plasma concentration is not a good criterion for assessing BDNF and may not show the BDNF changes. However, most animal studies were conducted on the expression of BDNF. The expression of genes and changes in the hippocampus prove the effects of exercises on BDNF changes. Exercise could positively affect BDNF levels in the plasma and hippocampus. The exact mechanism that shows the effects exercises on the changes of the factor has not been determined. However, exercises may help improve this factor's concentration or its expression by increasing angiogenesis and reducing inflammation and oxidative stress [44, 45]. AD is accompanied with stress and inflammation [46, 47]. Several studies have reported anti-inflammatory and antioxidant properties of exercises [48-50].
Thus, exercises can be considered a supplementary treatment for patients with AD. We recommend clinical studies on humans with the help of assessing the expression of BDNF to elucidate the exercises on BDNF concentration.
Conclusion
Exercise has positive effects on improving and increasing the expression and concentrations of Brain-Derived Neurotrophic Factor.
Acknowledgments: Not applicable.
Ethical Permissions: Not applicable.
Conflicts of Interests: The authors declare no competing interests in this work.
Authors’ Contribution: Rostami Mehr S (First Author), Introduction Writer/Methodologist/ Statistical Analyst (20%); Hossein Gholizadeh Salmani R (Second Author), Methodologist/Statistical Analyst (10%); Abbasi-Maleki S (Third Author), Methodologist/Discussion Writer (10%); Rasheed S (Fourth Author), Methodologist/Statistical Analyst (15%); Lorenzo-Villegas DL (Fifth Author), Methodologist/Discussion Writer (15%); Haghipanah M (Sixth Author), Methodologist/Main or Assistant/Discussion Writer/Statistical Analyst (30%)
Funding/Support: Not applicable.
Alzheimer's disease (AD) is a type of brain dysfunction that gradually degrades the patient's mental abilities [1]. It is normal for people to become a little forgetful as they age, but memory disorder progresses gradually in AD [2]. Alzheimer's causes the death of nerve cells and loss of tissue throughout the brain [3]. With the severity of the disease, the brain tissue shrinks, and the areas containing cerebrospinal fluid become larger [4].
The damage caused by Alzheimer's affects the affected person's memory, speech, and perception. Memory problems are usually one of the first symptoms of Alzheimer's [5]. However, the initial symptoms of this disease vary from person to person. Complications such as impaired performance in other aspects of thinking, such as finding the right words while speaking, visual/spatial problems, and impaired reasoning or judgment can be another sign of the early stages of AD [6, 7]. Memory problems are usually one of the first symptoms of Alzheimer's [8]. However, the initial symptoms of this disease vary from person to person. Complications such as impaired performance in other aspects of thinking, such as finding the right words while speaking, visual/spatial problems, and impaired reasoning or judgment can be another sign of the early stages of Alzheimer's disease [9, 10].
Alzheimer's disease affects the secretion of many neurotrophins [11]. These are nerve growth factors and are a type of cytokine. Neurotrophins, including Brain-Derived Neurotrophic Factor (BDNF), are key in increasing sympathetic activity and blood pressure [12, 13]. Studies have shown that the level of BDNF decreases in AD, and the decrease in this factor is consistent with the decrease in hippocampal volume in this disease [14, 15]. BDNF affects the intracellular signaling pathway of the proliferation of hippocampus cells and thus interferes in the development of AD [16, 17].
Alzheimer's has no cure, and there is no way to slow down the rate of nerve damage that it causes in the brain [18]. However, some treatments seem to help maintain mental skills and reduce the effects of the disease. On the other hand, physical exercises can protect against deterioration [19]. Increasing physical activity is effective in different lifestyles and increases brain activity, especially in the hippocampus area (memory center and learning), thus decreasing AD's secondary effects [20, 21]. It seems that the aerobic exercise program can effectively improve the neuropsychological functions of these patients. Several studies have reported the efficiency of exercises on BDNF in AD [22-24], but there is no comprehensive review on the effects of exercise on BDNF in AD. Thus, this study aimed to evaluate the effects of exercises on Brain-Derived Neurotrophic Factor levels in patients with Alzheimer's.
Information and Methods
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [25] in 2023. English papers on the effects of exercises on BDNF levels in subjects with Alzheimer's were included. Studies lacking complete data (e.g., lack of subjects, their numbers, periods, sample collection, and unclear results) were excluded. Unpublished papers, review papers, and studies with deficient information were not included. Papers were searched with keywords of “exercise” OR “BDNF” OR “neurotrophins” OR “rats” OR “mice” OR “humans”. The second strategy was to search reference lists in review articles for related articles. Articles were searched based on title and abstract. Duplicate papers were excluded, and potential papers were included. Searches were finalized on September 5th, 2023. Searches lasted for weeks to obtain final papers. Reviews and other mentioned non-related papers were excluded.
A standardized data coding form was prepared to extract the information from each study, including authors and publication year, study sample characteristics (number of subjects and their type), period of study, and results. Each study has only participated in the current research once [26]. In the current study, 12 articles were identified with the help of a database search and searching in a reference list. Keywords were searched in PubMed, Scopus, EuropePMC, Cochrane Central Database, Embase, and Web of Science databases (Figure 1). All the studies were managed with Endnote™ X9.2 (Thomson Reuters, Philadelphia, PA, USA) software. All the papers were read and investigated by two reviewers.

Figure 1. PRISMA flow diagram for included studies for the effects of exercises on BDNF
Findings
Twelve papers were reviewed, comprising 4 human and 8 animal studies. The periods for human studies lasted 26 to 52 weeks. Animal studies lasted 4 to 13 weeks. The samples were collected from plasma in human studies, while most animal studies were conducted on the expression of BDNF in the hippocampus (n=6). Out of 12 papers, 3 human papers and 8 animal studies showed that exercises significantly increased the concentration of the expression of BDNF (Table 1).
Table 1. Studies conducted on the effects of exercises on BDNF in subjects

Discussion
This systematic review assessed the effects of exercises on BDNF levels in subjects with Alzheimer's. Most human studies were conducted on middle-aged and elderly people. It is a common disease in elderly people. Alzheimer's disease in the elderly is a brain disorder that slowly destroys memory and thinking skills and, ultimately, the ability to do the simplest tasks. Recent estimates show that Alzheimer's disease is the third leading cause of death in the elderly after heart disease and cancer [39, 40].
Alzheimer's in the elderly is the most common cause of dementia among the elderly. Dementia is the loss of cognitive function-thinking, remembering, reasoning, and behavioral abilities to the extent that it interferes with a person's daily life and activities [41]. The severity of dementia ranges from the mildest stage, when it just begins to affect a person's functioning, to the most severe stage, when the person must be completely dependent on others for basic activities of daily living [42]. The damage occurs first in the hippocampus and entorhinal cortex, parts of the brain essential to forming memories. As more nerve cells die, other brain parts are affected and diminish. In the late stage of Alzheimer's in the elderly, the damage is extensive, and the brain tissue has significantly diminished [43].
Most studies on animal models were also conducted on elderly animals. Thus, animal models emphasize aged animals. The studies had differences for periods. Human studies were conducted for longer times, 26 weeks and longer. Since human studies were scarce, a relation between the period and efficiency of exercises can be stated. However, longer and shorter animal studies reported the positive effects. It can be stated that short-term exercises can increase BDNF in animal models. The results also showed that exercises influence the concentration of BDNF in the plasma. A study showed that exercises cannot positively affect the concentration plasma of BDNF [28]. The plasma concentration is not a good criterion for assessing BDNF and may not show the BDNF changes. However, most animal studies were conducted on the expression of BDNF. The expression of genes and changes in the hippocampus prove the effects of exercises on BDNF changes. Exercise could positively affect BDNF levels in the plasma and hippocampus. The exact mechanism that shows the effects exercises on the changes of the factor has not been determined. However, exercises may help improve this factor's concentration or its expression by increasing angiogenesis and reducing inflammation and oxidative stress [44, 45]. AD is accompanied with stress and inflammation [46, 47]. Several studies have reported anti-inflammatory and antioxidant properties of exercises [48-50].
Thus, exercises can be considered a supplementary treatment for patients with AD. We recommend clinical studies on humans with the help of assessing the expression of BDNF to elucidate the exercises on BDNF concentration.
Conclusion
Exercise has positive effects on improving and increasing the expression and concentrations of Brain-Derived Neurotrophic Factor.
Acknowledgments: Not applicable.
Ethical Permissions: Not applicable.
Conflicts of Interests: The authors declare no competing interests in this work.
Authors’ Contribution: Rostami Mehr S (First Author), Introduction Writer/Methodologist/ Statistical Analyst (20%); Hossein Gholizadeh Salmani R (Second Author), Methodologist/Statistical Analyst (10%); Abbasi-Maleki S (Third Author), Methodologist/Discussion Writer (10%); Rasheed S (Fourth Author), Methodologist/Statistical Analyst (15%); Lorenzo-Villegas DL (Fifth Author), Methodologist/Discussion Writer (15%); Haghipanah M (Sixth Author), Methodologist/Main or Assistant/Discussion Writer/Statistical Analyst (30%)
Funding/Support: Not applicable.
References
1. Bhushan I, Kour M, Kour G, Gupta S, Sharma S, Yadav A. Alzheimer's disease: Causes & treatment-A review. Ann Biotechnol. 2018;1(1):1002. [Link] [DOI:10.33582/2637-4927/1002]
2. Pradhan A, Gige J, Eliazer M. Detection of Alzheimer's disease (AD) in MRI images using deep learning. Int J Eng Res Technol. 2021;10(3):580-5. [Link]
3. Calvo-Rodriguez M, Bacskai BJ. Mitochondria and calcium in Alzheimer's disease: From cell signaling to neuronal cell death. Trends Neurosci. 2021;44(2):136-51. [Link] [DOI:10.1016/j.tins.2020.10.004]
4. Bergsland N, Dwyer MG, Jakimovski D, Weinstock‐Guttman B, Zivadinov R. Diffusion tensor imaging reveals greater microstructure damage in lesional tissue that shrinks into cerebrospinal fluid in multiple sclerosis. J Neuroimaging. 2021;31(5):995-1002. [Link] [DOI:10.1111/jon.12891]
5. Mirakhori F, Moafi M, Milanifard M, Tahernia H. Diagnosis and treatment methods in alzheimer's patients based on modern techniques: The orginal article. J PharmNegative Results. 2022;13(1):1889-907. [Link] [DOI:10.47750/pnr.2022.13.S01.226]
6. Van Crevel H. Clinical approach to dementia. Prog Brain Res. 1986;70:3-13. [Link] [DOI:10.1016/S0079-6123(08)64294-6]
7. Torregrossa W, Torrisi M, De Luca R, Casella C, Rifici C, Bonanno M, et al. Neuropsychological assessment in patients with traumatic brain injury: A comprehensive review with clinical recommendations. Biomedicines. 2023;11(7):1991. [Link] [DOI:10.3390/biomedicines11071991]
8. Chapman CA, Hasan O, Schulz PE, Martin RC. Evaluating the distinction between semantic knowledge and semantic access: Evidence from semantic dementia and comprehension-impaired stroke aphasia. Psychon Bull Rev. 2020;27(4)607-39. [Link] [DOI:10.3758/s13423-019-01706-6]
9. Barnard E. Neuropsychological assessment of driving abilities in patients with Alzheimer's disease or Parkinson's disease. Massachusetts: William James College; 2021. [Link]
10. Neel IC. Alzheimer's disease and other dementias, geriatric medicine: A person-centered evidence-based approach. New York: Springer; 2022. pp. 1-20. [Link] [DOI:10.1007/978-3-030-01782-8_84-1]
11. Ouerdane Y, El-Nahas ZS, Ouerdane F, Hamam KM, Ebada MA. Gut-brain axis in Alzheimer's disease: Interplay between cholecystokinin, dysbiosis, and brain-derived neurotrophic factor. In: Current thoughts on dementia: From risk factors to therapeutic interventions. New York: Springer; 2022. pp. 311-53. [Link] [DOI:10.1007/978-981-16-7606-2_12]
12. Sochal M, Ditmer M, Gabryelska A, Białasiewicz P. The role of brain-derived neurotrophic factor in immune-related diseases: A narrative review. J Clin Med. 2022;11(20):6023. [Link] [DOI:10.3390/jcm11206023]
13. Gabryelska A, Turkiewicz S, Ditmer M, Sochal M. Neurotrophins in the neuropathophysiology, course, and complications of obstructive sleep apnea-A narrative review. Int J Mol Sci. 2023;24(3):1808. [Link] [DOI:10.3390/ijms24031808]
14. Franzmeier N, Ren J, Damm A, Monté-Rubio G, Boada M, Ruiz A, et al. The BDNF Val66Met SNP modulates the association between beta-amyloid and hippocampal disconnection in Alzheimer's disease. Mol Psychiatry. 2021;26(2):614-28. [Link] [DOI:10.1038/s41380-019-0404-6]
15. Fang Y, Du N, Xing L, Duo Y, Zheng L. Evaluation of hippocampal volume and serum brain-derived neurotrophic factor as potential diagnostic markers of conversion from amnestic mild cognitive impairment to Alzheimer disease: A STROBE-compliant article. Medicine. 2019;98(30):e16604. [Link] [DOI:10.1097/MD.0000000000016604]
16. Gao L, Zhang Y, Sterling K, Song W. Brain-derived neurotrophic factor in Alzheimer's disease and its pharmaceutical potential. Transl Neurodegener. 2022;11(1):4. [Link] [DOI:10.1186/s40035-022-00279-0]
17. Zarneshan SN, Fakhri F, Khan H. Targeting Akt/CREB/BDNF signaling pathway by ginsenosides in neurodegenerative diseases: A mechanistic approach. Pharmacol Res. 12022;177:106099. [Link] [DOI:10.1016/j.phrs.2022.106099]
18. Li Q, Wu Y, Chen J, Xuan A, Wang X. Microglia and immunotherapy in Alzheimer's disease. Acta Neurologica Scandinavica. 2022;145(3):273-8. [Link] [DOI:10.1111/ane.13551]
19. Cunningham C, O'Sullivan R, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: A systematic review of reviews and meta‐analyses. Scand J Med Sci Sports. 2020;30(5):816-27. [Link] [DOI:10.1111/sms.13616]
20. Farì G, Lunetti P, Pignatelli G, Raele MV, Cera A, Mintrone G, et al. The effect of physical exercise on cognitive impairment in neurodegenerative disease: From pathophysiology to clinical and rehabilitative aspects. Int J Mol Sci. 2021;22(21):11632. [Link] [DOI:10.3390/ijms222111632]
21. Park SS, Park HS, Kim CJ, Kang HS, Kim DH, Baek SS, Kim TW. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer's disease. Alzheimer's Res Ther. 2020;12(62):1-15. [Link] [DOI:10.1186/s13195-020-00631-4]
22. Reycraft JT, Islam H, Townsend LK, Hayward GC, Hazell TJ, MacPherson RE. Exercise intensity and recovery on circulating brain-derived neurotrophic factor. Med Sci Sports Exerc. 2020;52(2):1210-7. [Link] [DOI:10.1249/MSS.0000000000002242]
23. García-Mesa Y, Pareja-Galeano H, Bonet-Costa V, Revilla S, Gómez-Cabrera MC, Gambini J, et al. Physical exercise neuroprotects ovariectomized 3xTg-AD mice through BDNF mechanisms. Psychoneuroendocrinology. 2014;45:154-66. [Link] [DOI:10.1016/j.psyneuen.2014.03.021]
24. Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science. 2018;361(6406):eaan8821. [Link] [DOI:10.1126/science.aan8821]
25. Moher D, Liberati A, Tetzlaff J, Altman DG. Research methods & reporting-preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement-David Moher and colleagues introduce PRISMA, an update of the QUOROM guidelines for reporting systematic reviews and meta-analyses. BMJ. 2009;339:b2535. [Link] [DOI:10.1136/bmj.b2535]
26. Cooper H. Research synthesis and meta-analysis: A step-by-step approach. New York: Sage publications; 2015. [Link]
27. de Melo Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R,et al. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer's disease. J Alzheimers Dis. 2014;39(2):401-8. [Link] [DOI:10.3233/JAD-131073]
28. Gaitán JM, Moon HY, Stremlau M, Dubal DB, Cook DB, Okonkwo OC, et al. Effects of aerobic exercise training on systemic biomarkers and cognition in late middle-aged adults at risk for Alzheimer's disease. Front Endocrinol. 2021;12:660181. [Link] [DOI:10.3389/fendo.2021.660181]
29. Nascimento CMC, Rodrigues Pereira J, Pires de Andrade L, Garuffi M, Leme Talib L, Vicente Forlenza O, et al. Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Curr Alzheimer Res. 2014;11(8):799-805. [Link] [DOI:10.2174/156720501108140910122849]
30. Håkansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, et al. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: Associations with working memory function. J Alzheimers Dis. 2017;55(2);645-57. [Link] [DOI:10.3233/JAD-160593]
31. Koo JH, Kwon IS, Kang EB, Lee CK, Lee NH, Kwon MG, et al. Neuroprotective effects of treadmill exercise on BDNF and PI3-K/Akt signaling pathway in the cortex of transgenic mice model of Alzheimer's disease. J Exerc Nutrition Biochem. 2013;17():151. [Link] [DOI:10.5717/jenb.2013.17.4.151]
32. Naghibi S, Joneydi MS, Barzegari A, Davoodabadi A, Ebrahimi A, Eghdami E, et al. Treadmill exercise sex-dependently alters susceptibility to depression-like behaviour, cytokines and BDNF in the hippocampus and prefrontal cortex of rats with sporadic Alzheimer-like disease. Physiol Behav. 2021;241:113595. [Link] [DOI:10.1016/j.physbeh.2021.113595]
33. Özbeyli D, Sarı G, Özkan N, Karademir B, Yüksel M, Kaya OTC, et al. Protective effects of different exercise modalities in an Alzheimer's disease-like model. Behav Brain Res. 2017;328:159-77. [Link] [DOI:10.1016/j.bbr.2017.03.044]
34. Dao AT, Zagaar MA, Levine AT, Salim S, Eriksen JL, Alkadhi KA. Treadmill exercise prevents learning and memory impairment in Alzheimer's disease-like pathology. Curr Alzheimer Res. 2013;10(5):507-15. [Link] [DOI:10.2174/1567205011310050006]
35. Sim YJ. Treadmill exercise alleviates impairment of spatial learning ability through enhancing cell proliferation in the streptozotocin-induced Alzheimer's disease rats. J Exerc Rehabil. 2014;10(2):81-5. [Link] [DOI:10.12965/jer.140102]
36. Alkadhi KA, Dao AT. Exercise decreases BACE and APP levels in the hippocampus of a rat model of Alzheimer's disease. Mol Cell Neurosci. 2018;86:25-9. [Link] [DOI:10.1016/j.mcn.2017.11.008]
37. Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10(1):2-8. [Link] [DOI:10.12965/jer.140086]
38. Lin TW, Shih YH, Chen SJ, Lien CH, Chang CY, Huang TY, et al. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer's disease (APP/PS1) transgenic mice. Neurobiol Learn Memory. 2015;118:189-97. [Link] [DOI:10.1016/j.nlm.2014.12.005]
39. A.s. Association. 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11(3):332-84. [Link] [DOI:10.1016/j.jalz.2015.02.003]
40. A.s. Association. 2017 Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2017;13():325-73. [Link] [DOI:10.1016/j.jalz.2017.02.001]
41. Madhavan J, Anagha P, Vinod R, Aparna P. Dementia associated with seizure disorder-A case report. J Ayurveda Integrated Med Sci. 2020;5(2):282-7. [Link]
42. Abreu W, Tolson D, Jackson GA, Staines H, Costa N. The relationship between frailty, functional dependence, and healthcare needs among community‐dwelling people with moderate to severe dementia. Health Soc Care Community. 2019;27(3):642-53. [Link] [DOI:10.1111/hsc.12678]
43. Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35(22):7497-504. [Link] [DOI:10.1093/nar/gkm821]
44. El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013;65:380-401. [Link] [DOI:10.1016/j.freeradbiomed.2013.07.003]
45. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reproductive Biol Endocrinol. 2012;10(49):1-31. [Link] [DOI:10.1186/1477-7827-10-49]
46. Pasinetti GM. Cyclooxygenase and inflammation in Alzheimer's disease: Experimental approaches and clinical interventions. J Neurosci Res. 1998;54(1):1-6.
https://doi.org/10.1002/(SICI)1097-4547(19981001)54:1<1::AID-JNR1>3.0.CO;2-M [Link] [DOI:10.1002/(SICI)1097-4547(19981001)54:13.0.CO;2-M]
47. Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, et al. α-lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535-48. [Link] [DOI:10.1016/j.redox.2017.11.001]
48. Daniela M, Catalina L, Ilie O, Paula M, Daniel-Andrei I, Ioana B. Effects of exercise training on the autonomic nervous system with a focus on anti-inflammatory and antioxidants effects. Antioxidants. 2022;11(2):350. [Link] [DOI:10.3390/antiox11020350]
49. Villarreal-Soto SA, Beaufort S, Bouajila J, Souchard JP, Renard T, Rollan S, Taillandier P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019;83:44-54. [Link] [DOI:10.1016/j.procbio.2019.05.004]
50. Magrone T, Magrone M, Russo MA, Jirillo E. Recent advances on the anti-inflammatory and antioxidant properties of red grape polyphenols: In vitro and in vivo studies. Antioxidants. 2019;9(1):35. [Link] [DOI:10.3390/antiox9010035]









