GMJ Medicine
eISSN : 2626-3041
Volume 4, Issue 2 (2025)
GMJM 2025, 4(2): 77-82 |
Back to browse issues page
Article Type:
Subject:
History
Received: 2024/11/15 | Accepted: 2025/05/1 | Published: 2025/06/7
Received: 2024/11/15 | Accepted: 2025/05/1 | Published: 2025/06/7
How to cite this article
Abbasi Maleki S, Gholami M, Ghazanfari Hashemi M, Hossein Gholizadeh Salmani R, Moradikor N. Protective Effects of Black Cumin Nanophytosome in Rats Exposed to Chronic Stress. GMJM 2025; 4 (2) :77-82
URL: http://gmedicine.de/article-2-263-en.html
URL: http://gmedicine.de/article-2-263-en.html
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



Rights and permissions
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Authors
S. Abbasi Maleki1, M. Gholami2, M. Ghazanfari Hashemi3, R. Hossein Gholizadeh Salmani4, N. Moradikor *5
1- Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
2- Department of Pharmacology & Toxicology, Faculty of Pharmacy and Pharmaceutical Sciences, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran
3- School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
4- Department of Pathobiology, Faculty of Veterinary Medicine, Urmia Branch, Islamic Azad University, Urmia, Iran
5- International Center for Neuroscience Research, Institute for Intelligent Research, Tbilisi, Georgia
2- Department of Pharmacology & Toxicology, Faculty of Pharmacy and Pharmaceutical Sciences, Tehran Medical Sciences Branch, Islamic Azad University, Tehran, Iran
3- School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
4- Department of Pathobiology, Faculty of Veterinary Medicine, Urmia Branch, Islamic Azad University, Urmia, Iran
5- International Center for Neuroscience Research, Institute for Intelligent Research, Tbilisi, Georgia
Keywords:
Anxiety [MeSH], Antioxidant [MeSH], Brain-Derived Neurotrophic Factor [MeSH], Cumin [MeSH], Nanophytosome [MeSH], Stress [MeSH]
| Abstract (HTML) (1809 Views)
Full-Text: (648 Views)
Introduction
Humans and animals usually experience stress during their lives. Stress can be grouped into acute and chronic stresses. Acute stress is usually observed in emergencies such as fighting or escaping [1]. Changes in the structure and function of molecules and tissues in the brain region induce the emotional and cognitive system to make decisions concerning stress-coping mechanisms [2]. Prolonged exposure to stress causes the development of psychiatric conditions such as anxiety disorders and depression, which causes aberrant amygdala activation and deficits in emotion and behavior [3]. Psychiatric symptoms concerning the core neurobiological properties across stress-associated neuropsychiatric disorders have been recognized [4]. Prolonged stress suppresses cell proliferation and decreases neurogenesis which plays an important role in the pathogenesis of depression and anxiety [5]. Chronic stresses influence neurogenesis and impairment of neurotransmission by affecting immune and hormonal effects [6]. Brain-derived neurotrophic factor (BDNF) is essential in synaptic plasticity and neuronal survival [7]. It has an important role in improving normal and faulted memory functions. Its levels are decreased during chronic stress conditions [8]. Stress disturbs the antioxidant-oxidant system. Antioxidants can decrease and inhibit the destructive effects of free radicals on cells [5]. Plants and their derivations are rich sources of antioxidants that can be used to prevent and treat stress.
Black cumin is a plant used for pharmaceutical uses. The traditional uses of cumin seeds are due to their medicinal properties such as antioxidant, anti-inflammatory, immunomodulatory, anticancer, neuroprotective, antimicrobial, antihypertensive, and hepatoprotective properties [9]. It contains some compounds with pharmacological properties, such as thymoquinone, thymohydroquinone, thymol, carvacrol, nigellidine, and α-hederin [10]. Several studies have reported the antioxidant activity of cumin [11-13]. Studies have reported positive effects of cumin on anxiety and depression in different models [14-16]. However, cumin essential oil is volatile and can be easily degraded. There is a need to coat it with safe agents.
Phytosome is a chemical interaction between phospholipid molecule and polyphenolic compound via hydrogen bond formation and/or van der Waal attraction force. The physicochemical stability of pyrosomes relies on the physicochemical characteristics of the drug-lipid complex, such as phase transition temperature, solubility, melting point, and lipid composition, which results in better physical stability over conventional liposomes [17].
Cumin may decrease behavioral responses and biochemical factors positively due to its antioxidant activity. Coating it with phytosom protects it against degradation. However, we could not find any study evaluating the effects of black cumin nanophytosome on behavioral responses, stress oxidative biochemical factors, and BDNF, a chronic stress model. This study aimed to evaluate the effects of black cumin nanophytosomes on behavioral responses, stress oxidative biochemical factors, and BDNF changes in rats exposed to chronic stress.
Materials and Methods
In this experimental study, 60 four-week Wistar female pup rats (45±5g) were divided into six groups (10 rats each). The rats were grouped into six groups of non-stressed (negative control or NC) and stressed treated with 0mg/kg of cumin essential oil (Stress) and 20mg/kg of cumin (20-C), 40mg/kg of cumin (40-C), 20mg/kg of cumin nanophytosome (20-CN) and 40mg/kg of cumin nanophytosome (40-CN).
To evaluate the anxiety and depression, an Open-Field Test (OFT) with the help of a dark area (72×72×45cm), an Elevated Plus Maze (EPM) test, and a Force Swimming Test (FST) with the help of a cylindrical swimming tank were used [20]. The animals were decapitated, and the whole prefrontal cortex (PFC) was dissected and then immediately frozen at -80°C. The samples were homogenously prepared and assessed with Rat BDNF ELISA kits (Hangzhou Eastbiopharm Co., LTP; China). Ferric-reducing antioxidant power (FRAP) and malondialdehyde (MDA) were assessed in brain samples [20]. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were measured in the supernatant with ZellBio GmbH, Ulm kits (Zellbio; Germany).
The rats were prepared and kept under 12h light/dark cycle and in a controlled room temperature (22±2°C) with free access to food and water. Thirty rats were orally administered 10, 20, 30, 40, and 50mg/kg of cumin essential oil (Barij Essence; Iran) and its nanophytosome for 28 days [18]. Clinical signs, toxicity, and mortality were recorded 24h after the last administration. No toxicity was observed, and doses of 20mg/kg and 40mg/kg were selected. Stress was induced with a clear polyethylene cylinder [19].
The data were analyzed with the help of ANOVA pathway for comparison between groups. Duncan test was used as post-hoc test for comparison between groups. The data were reported as mean±SD.
Findings
Anxiety-like behaviors
The stress decreased the number of visits (p=0.001), center time (p=0.001), and total distance (p=0.001) compared with control group rats. However, the treatment with bare cumin and coated cumin in a dose-dependent manner increased the number of visits (p=0.001), center time (p=0.001), and total distance (p=0.001) compared with the stress group rats. The highest number of visits, center time, and total distance in stressed rats were observed in rats treated with 40mg of cumin nanophytosomes (Figure 1).
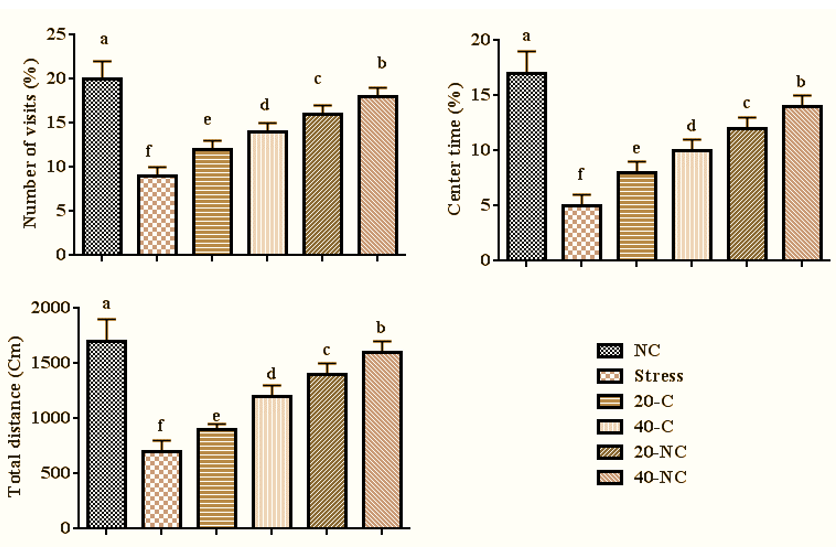
Figure 1. Anxiety-like behaviors in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups
Anxiety-like parameters
The stress had negative effects on anxiety-like parameters and reduced open arm time (p=0.001), and open arm entrance (p=0.001) compared with control group rats. Oral gavage of cumin in a dose-dependent manner and especially in a nanophytosome manner increased open arm time (p=0.001), and open arm entrance (p=0.001) compared with rats in the stress group (Figure 2).
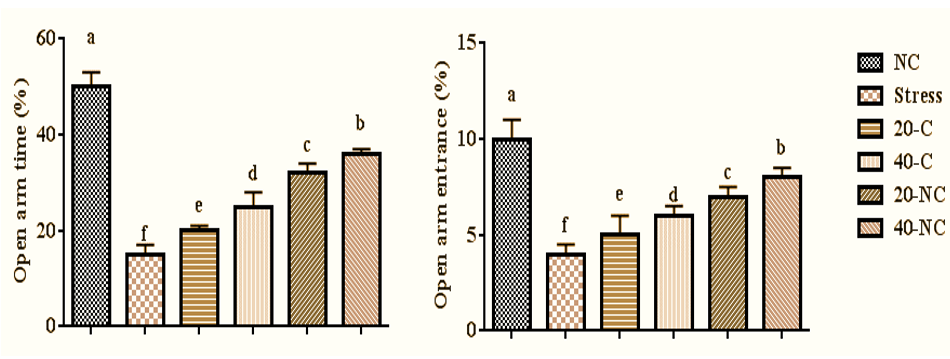
Figure 2. Anxiety-like parameters in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Depression-like behaviors
The stress decreased swimming duration (p=0.001), and increased immobility time (p=0.001) compared with control group rats. However, the treatment with bare cumin and coated cumin in a dose-dependent manner increased swimming duration (p=0.001), and decreased immobility time (p=0.001) compared with rats in the stress group. The highest values for swimming duration and lowest values for immobility time were seen in rats treated with 40mg of cumin nanophytosome (Figure 3).
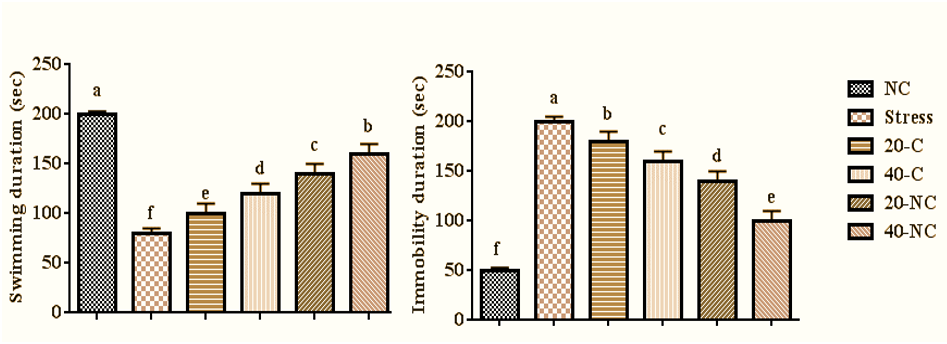
Figure 3. Depression-like behaviors in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Prefrontal cortex BDNF levels
The stress decreased prefrontal cortex BDNF levels compared with non-stressed rats (p=0.001). Administration of cumin in a dose-dependent manner increased BDNF. The highest BDNF levels in stressed rats were observed in rats treated with 40mg of cumin nanophytosomes (Figure 4).
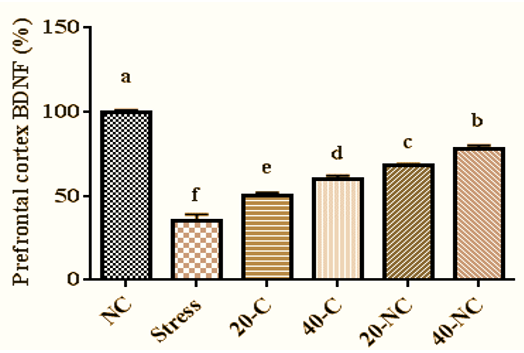
Figure 4. Prefrontal cortex BDNF levels in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Oxidative stress markers and antioxidant enzyme activity
The stress reduced FRAP concentration (p=0.001), SOD (p=0.001), and GPx (p=0.001) and increased MDA (p=0.001) compared with control group rats. However, the treatment with bare cumin and coated cumin in a dose-dependent manner increased FRAP concentration (p=0.001), SOD (p=0.001), and GPx (p=0.001) and decreased MDA (p=0.001) compared with rats in the stress group. The highest values for FRAP concentration, SOD, and GPx were seen in rats treated with 40mg of cumin nanophytosome (Figure 5).
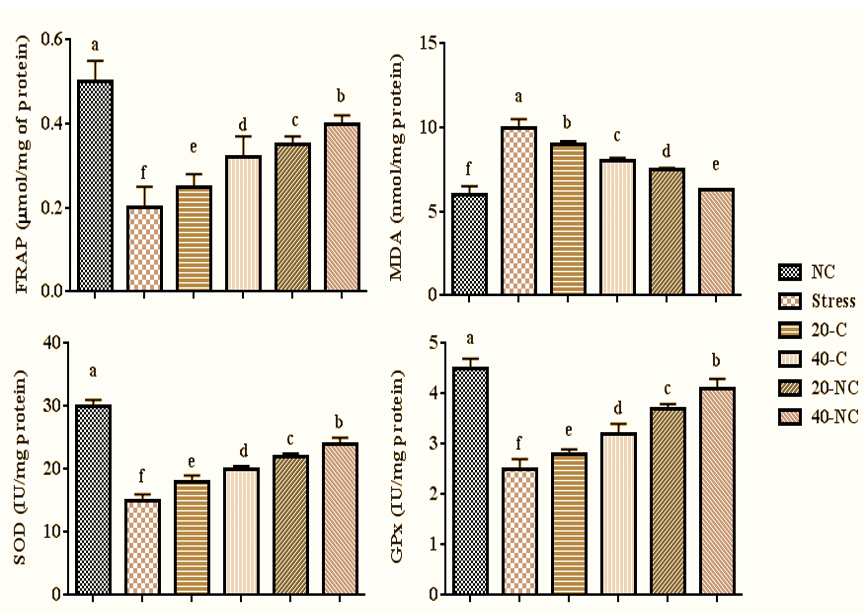
Figure 5. Oxidative stress markers and antioxidant enzymes activity in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Discussion
This study aimed to evaluate the effects of black cumin nanophytosomes on behavioral responses, stress oxidative biochemical factors, and BDNF in rats exposed to chronic stress. The results showed that stress had negative effects on behavioral responses. It induced depression and anxiety-like behaviors in the rats. The results for the effects of stress on anxiety and depression are in agreement with previous studies [21-23].
Chronic stress was reported to decrease BDNF levels and mRNA expression in the amygdala. They claimed that a decrease in expression and concentration of BDNF and its receptor in the amygdala are risk factors for anxiety of depressive-like behaviors [19, 20]. Indeed, the amygdala is a limbic structure that is involved in emotion and changes its structure and function after chronic stress which may participate in mood disorders like depression and anxiety. This study was conducted on rats at low ages, which can influence their mood at higher ages. Studies have shown that BDNF is a major neurotrophic factor in the brain that increases neuronal viability during development and adulthood [19]. Our findings show that stress significantly decreased BDNF levels. It means that stress has negative effects on the brain. It can be stated that BDNF and its receptor may be considered as a target for the negative effects of stress on the amygdala that cause faults in the amygdala and affect the development of depression-like behaviors in adulthood. We also believe that stress affects oxidant and antioxidant balance, which influence anxiety and depression. Our results showed that stress decreased the activities of antioxidant enzymes while increasing the concentration of MDA. The results agree with other studies on the effects of stress on the activities of antioxidant enzymes [24]. Indeed, MDA is a final product of peroxidation and has a negative relation with the antioxidant system. In the current study, the antioxidant activity of enzymes was decreased under stress conditions. Stress induces damage in the brain and causes anxiety and depression. It can be stated that stress induces damage in terms of anxiety and depression by disturbing the antioxidant-oxidant system.
The administration of cumin essential oil in a dose-dependent manner decreased anxiety and depression-like effects which is in agreement with other studies [14-16]. As mentioned in the previous section, stress affects behaviors by influencing BDNF and the antioxidant-oxidant system. The results showed that administration of cumin essential increased BDNF levels which is in agreement with other studies [10, 25]. Although cumin increased BDNF levels its mechanism is unknown. Cumin essential oil seems to influence the brain regions involved in the expression of BDNF. On the other hand, cumin improved antioxidant properties which agree with previous studies [11-13]. We believe that cumin keeps GPx and SOD activities and prevents decreased antioxidant enzymes. Indeed, cumin keeps antioxidant enzyme activities. The increase in the activity of antioxidant enzymes reduces MDA concentration. Higher concentrations of cumin had higher effects which could be attributed to the effects of its active compounds. In addition, coating cumin with nanophytosomes had higher effects, which could be attributed to the effects of phytosomes in the prevention of degradation of the essential oil. This study was conducted on rats, a major limitation of our study.
Conclusion
The treatment with cumin in coated form and at the highest concentrations (especially at 40mg/kg nanophytosome cumin essential oil) decreases depression and anxiety-like behaviors by increasing BDNF and antioxidant factors.
Acknowledgments: We want to thank CASRP for the English editing.
Ethical Permissions: The procedures used were confirmed by the Ethics Committee of the International Center for Neuroscience Research (GE-ICNR-2021-10048).
Conflicts of Interests: The authors declare no competing interests in this work.
Authors’ Contribution: Abbasi Maleki S (First Author), Introduction Writer/Methodologist/ Main Researcher/Statistical Analyst (20%); Gholami M (Second Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Ghazanfari Hashemi M (Third Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Hossein Gholizadeh Salmani R (Fourth Author), Methodologist/Assistant Researcher (10%); Moradikor N (Fifth Author), Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (40%)
Funding/Support: The authors gratefully acknowledge the International Center for Neuroscience Research staff for their cooperation and support.
Humans and animals usually experience stress during their lives. Stress can be grouped into acute and chronic stresses. Acute stress is usually observed in emergencies such as fighting or escaping [1]. Changes in the structure and function of molecules and tissues in the brain region induce the emotional and cognitive system to make decisions concerning stress-coping mechanisms [2]. Prolonged exposure to stress causes the development of psychiatric conditions such as anxiety disorders and depression, which causes aberrant amygdala activation and deficits in emotion and behavior [3]. Psychiatric symptoms concerning the core neurobiological properties across stress-associated neuropsychiatric disorders have been recognized [4]. Prolonged stress suppresses cell proliferation and decreases neurogenesis which plays an important role in the pathogenesis of depression and anxiety [5]. Chronic stresses influence neurogenesis and impairment of neurotransmission by affecting immune and hormonal effects [6]. Brain-derived neurotrophic factor (BDNF) is essential in synaptic plasticity and neuronal survival [7]. It has an important role in improving normal and faulted memory functions. Its levels are decreased during chronic stress conditions [8]. Stress disturbs the antioxidant-oxidant system. Antioxidants can decrease and inhibit the destructive effects of free radicals on cells [5]. Plants and their derivations are rich sources of antioxidants that can be used to prevent and treat stress.
Black cumin is a plant used for pharmaceutical uses. The traditional uses of cumin seeds are due to their medicinal properties such as antioxidant, anti-inflammatory, immunomodulatory, anticancer, neuroprotective, antimicrobial, antihypertensive, and hepatoprotective properties [9]. It contains some compounds with pharmacological properties, such as thymoquinone, thymohydroquinone, thymol, carvacrol, nigellidine, and α-hederin [10]. Several studies have reported the antioxidant activity of cumin [11-13]. Studies have reported positive effects of cumin on anxiety and depression in different models [14-16]. However, cumin essential oil is volatile and can be easily degraded. There is a need to coat it with safe agents.
Phytosome is a chemical interaction between phospholipid molecule and polyphenolic compound via hydrogen bond formation and/or van der Waal attraction force. The physicochemical stability of pyrosomes relies on the physicochemical characteristics of the drug-lipid complex, such as phase transition temperature, solubility, melting point, and lipid composition, which results in better physical stability over conventional liposomes [17].
Cumin may decrease behavioral responses and biochemical factors positively due to its antioxidant activity. Coating it with phytosom protects it against degradation. However, we could not find any study evaluating the effects of black cumin nanophytosome on behavioral responses, stress oxidative biochemical factors, and BDNF, a chronic stress model. This study aimed to evaluate the effects of black cumin nanophytosomes on behavioral responses, stress oxidative biochemical factors, and BDNF changes in rats exposed to chronic stress.
Materials and Methods
In this experimental study, 60 four-week Wistar female pup rats (45±5g) were divided into six groups (10 rats each). The rats were grouped into six groups of non-stressed (negative control or NC) and stressed treated with 0mg/kg of cumin essential oil (Stress) and 20mg/kg of cumin (20-C), 40mg/kg of cumin (40-C), 20mg/kg of cumin nanophytosome (20-CN) and 40mg/kg of cumin nanophytosome (40-CN).
To evaluate the anxiety and depression, an Open-Field Test (OFT) with the help of a dark area (72×72×45cm), an Elevated Plus Maze (EPM) test, and a Force Swimming Test (FST) with the help of a cylindrical swimming tank were used [20]. The animals were decapitated, and the whole prefrontal cortex (PFC) was dissected and then immediately frozen at -80°C. The samples were homogenously prepared and assessed with Rat BDNF ELISA kits (Hangzhou Eastbiopharm Co., LTP; China). Ferric-reducing antioxidant power (FRAP) and malondialdehyde (MDA) were assessed in brain samples [20]. Superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities were measured in the supernatant with ZellBio GmbH, Ulm kits (Zellbio; Germany).
The rats were prepared and kept under 12h light/dark cycle and in a controlled room temperature (22±2°C) with free access to food and water. Thirty rats were orally administered 10, 20, 30, 40, and 50mg/kg of cumin essential oil (Barij Essence; Iran) and its nanophytosome for 28 days [18]. Clinical signs, toxicity, and mortality were recorded 24h after the last administration. No toxicity was observed, and doses of 20mg/kg and 40mg/kg were selected. Stress was induced with a clear polyethylene cylinder [19].
The data were analyzed with the help of ANOVA pathway for comparison between groups. Duncan test was used as post-hoc test for comparison between groups. The data were reported as mean±SD.
Findings
Anxiety-like behaviors
The stress decreased the number of visits (p=0.001), center time (p=0.001), and total distance (p=0.001) compared with control group rats. However, the treatment with bare cumin and coated cumin in a dose-dependent manner increased the number of visits (p=0.001), center time (p=0.001), and total distance (p=0.001) compared with the stress group rats. The highest number of visits, center time, and total distance in stressed rats were observed in rats treated with 40mg of cumin nanophytosomes (Figure 1).

Figure 1. Anxiety-like behaviors in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups
Anxiety-like parameters
The stress had negative effects on anxiety-like parameters and reduced open arm time (p=0.001), and open arm entrance (p=0.001) compared with control group rats. Oral gavage of cumin in a dose-dependent manner and especially in a nanophytosome manner increased open arm time (p=0.001), and open arm entrance (p=0.001) compared with rats in the stress group (Figure 2).

Figure 2. Anxiety-like parameters in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Depression-like behaviors
The stress decreased swimming duration (p=0.001), and increased immobility time (p=0.001) compared with control group rats. However, the treatment with bare cumin and coated cumin in a dose-dependent manner increased swimming duration (p=0.001), and decreased immobility time (p=0.001) compared with rats in the stress group. The highest values for swimming duration and lowest values for immobility time were seen in rats treated with 40mg of cumin nanophytosome (Figure 3).

Figure 3. Depression-like behaviors in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Prefrontal cortex BDNF levels
The stress decreased prefrontal cortex BDNF levels compared with non-stressed rats (p=0.001). Administration of cumin in a dose-dependent manner increased BDNF. The highest BDNF levels in stressed rats were observed in rats treated with 40mg of cumin nanophytosomes (Figure 4).

Figure 4. Prefrontal cortex BDNF levels in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Oxidative stress markers and antioxidant enzyme activity
The stress reduced FRAP concentration (p=0.001), SOD (p=0.001), and GPx (p=0.001) and increased MDA (p=0.001) compared with control group rats. However, the treatment with bare cumin and coated cumin in a dose-dependent manner increased FRAP concentration (p=0.001), SOD (p=0.001), and GPx (p=0.001) and decreased MDA (p=0.001) compared with rats in the stress group. The highest values for FRAP concentration, SOD, and GPx were seen in rats treated with 40mg of cumin nanophytosome (Figure 5).

Figure 5. Oxidative stress markers and antioxidant enzymes activity in stressed rats treated with cumin and its nanophytsome. Different letters (a-f) show significant differences between groups.
Discussion
This study aimed to evaluate the effects of black cumin nanophytosomes on behavioral responses, stress oxidative biochemical factors, and BDNF in rats exposed to chronic stress. The results showed that stress had negative effects on behavioral responses. It induced depression and anxiety-like behaviors in the rats. The results for the effects of stress on anxiety and depression are in agreement with previous studies [21-23].
Chronic stress was reported to decrease BDNF levels and mRNA expression in the amygdala. They claimed that a decrease in expression and concentration of BDNF and its receptor in the amygdala are risk factors for anxiety of depressive-like behaviors [19, 20]. Indeed, the amygdala is a limbic structure that is involved in emotion and changes its structure and function after chronic stress which may participate in mood disorders like depression and anxiety. This study was conducted on rats at low ages, which can influence their mood at higher ages. Studies have shown that BDNF is a major neurotrophic factor in the brain that increases neuronal viability during development and adulthood [19]. Our findings show that stress significantly decreased BDNF levels. It means that stress has negative effects on the brain. It can be stated that BDNF and its receptor may be considered as a target for the negative effects of stress on the amygdala that cause faults in the amygdala and affect the development of depression-like behaviors in adulthood. We also believe that stress affects oxidant and antioxidant balance, which influence anxiety and depression. Our results showed that stress decreased the activities of antioxidant enzymes while increasing the concentration of MDA. The results agree with other studies on the effects of stress on the activities of antioxidant enzymes [24]. Indeed, MDA is a final product of peroxidation and has a negative relation with the antioxidant system. In the current study, the antioxidant activity of enzymes was decreased under stress conditions. Stress induces damage in the brain and causes anxiety and depression. It can be stated that stress induces damage in terms of anxiety and depression by disturbing the antioxidant-oxidant system.
The administration of cumin essential oil in a dose-dependent manner decreased anxiety and depression-like effects which is in agreement with other studies [14-16]. As mentioned in the previous section, stress affects behaviors by influencing BDNF and the antioxidant-oxidant system. The results showed that administration of cumin essential increased BDNF levels which is in agreement with other studies [10, 25]. Although cumin increased BDNF levels its mechanism is unknown. Cumin essential oil seems to influence the brain regions involved in the expression of BDNF. On the other hand, cumin improved antioxidant properties which agree with previous studies [11-13]. We believe that cumin keeps GPx and SOD activities and prevents decreased antioxidant enzymes. Indeed, cumin keeps antioxidant enzyme activities. The increase in the activity of antioxidant enzymes reduces MDA concentration. Higher concentrations of cumin had higher effects which could be attributed to the effects of its active compounds. In addition, coating cumin with nanophytosomes had higher effects, which could be attributed to the effects of phytosomes in the prevention of degradation of the essential oil. This study was conducted on rats, a major limitation of our study.
Conclusion
The treatment with cumin in coated form and at the highest concentrations (especially at 40mg/kg nanophytosome cumin essential oil) decreases depression and anxiety-like behaviors by increasing BDNF and antioxidant factors.
Acknowledgments: We want to thank CASRP for the English editing.
Ethical Permissions: The procedures used were confirmed by the Ethics Committee of the International Center for Neuroscience Research (GE-ICNR-2021-10048).
Conflicts of Interests: The authors declare no competing interests in this work.
Authors’ Contribution: Abbasi Maleki S (First Author), Introduction Writer/Methodologist/ Main Researcher/Statistical Analyst (20%); Gholami M (Second Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Ghazanfari Hashemi M (Third Author), Methodologist/Assistant Researcher/Statistical Analyst (15%); Hossein Gholizadeh Salmani R (Fourth Author), Methodologist/Assistant Researcher (10%); Moradikor N (Fifth Author), Methodologist/Main Researcher/Discussion Writer/Statistical Analyst (40%)
Funding/Support: The authors gratefully acknowledge the International Center for Neuroscience Research staff for their cooperation and support.
References
1. Sandi C, Haller J. Stress and the social brain: Behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290-304. [Link] [DOI:10.1038/nrn3918]
2. Dai S, Mo Y, Wang Y, Xiang B, Liao Q, Zhou M, et al. Chronic stress promotes cancer development. Front Oncol. 2020;10:1492. [Link] [DOI:10.3389/fonc.2020.01492]
3. Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat commu. 2020;11(1):2221. [Link] [DOI:10.1038/s41467-020-15920-7]
4. Robinson OJ, Krimsky M, Lieberman L, Allen P, Vytal K, Grillon C. Towards a mechanistic understanding of pathological anxiety: The dorsal medial prefrontal-amygdala 'aversive amplification'circuit in unmedicated generalized and social anxiety disorders. Lancet Psychiatry. 2014;1(4):294. [Link] [DOI:10.1016/S2215-0366(14)70305-0]
5. Juszczyk G, Mikulska J, Kasperek K, Pietrzak D, Mrozek W, Herbet M. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and Alzheimer's disease: The role of antioxidants in prevention and treatment. Antioxidants. 2021;10(9):1439. [Link] [DOI:10.3390/antiox10091439]
6. Jesulola E, Micalos P, Baguley IJ. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model-are we there yet?. Behav Brain Res. 2018;341:79-90. [Link] [DOI:10.1016/j.bbr.2017.12.025]
7. Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 2014;76:677-83. [Link] [DOI:10.1016/j.neuropharm.2013.04.024]
8. Alzoubi KH, Abdel-Hafiz L, Khabour OF, El-Elimat T, Alzubi MA, Alali FQ. Evaluation of the effect of Hypericum triquetrifolium turra on memory impairment induced by chronic psychosocial stress in rats: Role of BDNF. Drug Des Devel. 2020;14:5299-314. [Link] [DOI:10.2147/DDDT.S278153]
9. Yimer EM, Tuem KB, Karim A, Ur-Rehman N, Anwar F. Nigella sativa L. (Black Cumin): A promising natural remedy for wide range of illnesses. Evid Based Complement Alternat Med. 2019;2019:1528635. [Link] [DOI:10.1155/2019/1528635]
10. Hannan MA, Rahman MA, Sohag AAM, Uddin MJ, Dash R, Sikder MH, et al. Black Cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients. 2021;13(6):1784. [Link] [DOI:10.3390/nu13061784]
11. Amiri A, Mousakhani-Ganjeh A, Amiri Z, Guo YG, Singh AP, Kenari RE. Fabrication of cumin loaded-chitosan particles: Characterized by molecular, morphological, thermal, antioxidant and anticancer properties as well as its utilization in food system. Food Chem. 2020;310:125821. [Link] [DOI:10.1016/j.foodchem.2019.125821]
12. Mughal SS. A review on potential antioxidant effects of Cumin (Cuminum cyminum), phytochemical profile and its uses. GSJ. 2021;8(9):1-19. [Link] [DOI:10.22541/au.166401164.45578619/v1]
13. Gueffai A, Gonzalez-Serrano DJ, Christodoulou MC, Orellana-Palacios JC, Ortega MLS, Ouldmoumna A, et al. Phenolics from defatted black cumin seeds (Nigella sativa L.): Ultrasound-assisted extraction optimization, comparison, and antioxidant activity. Biomolecules. 2022;12(9):1311. [Link] [DOI:10.3390/biom12091311]
14. Cañedo-Ayala M, Rice DB, Levis B, Carrier ME, Cumin J, Malcarne VL, et al. Scleroderma Caregiver Advisory Committee. Factors associated with symptoms of depression among informal caregivers of people with systemic sclerosis: A cross-sectional study. Disabil Rehabil. 42(3):394-99. [Link] [DOI:10.1080/09638288.2018.1500647]
15. Kannan R, George S, Chakrapani BPS, Maliakel B, Ittiyavirah S, Krishnakumar I. Thymoquinone-rich black cumin oil improves sleep quality, alleviates anxiety/stress on healthy subjects with sleep disturbances-A pilot polysomnography study. J Herb Med. 2022;32:100507. [Link] [DOI:10.1016/j.hermed.2021.100507]
16. Ittiyavirah SP, Ramalingam K, Sathyan A, Rajasree R, Kuruniyan MS, Quadri SA, et al. Thymoquinone-rich black cumin oil attenuates ibotenic acid-induced excitotoxicity through glutamate receptors in Wistar rats. Saudi Pharm J. 2022;30(12):1781-90. [Link] [DOI:10.1016/j.jsps.2022.10.007]
17. Komeil IA, Abdallah OY, El-Refaie WM. Surface modified genistein phytosome for breast cancer treatment: In-vitro appraisal, pharmacokinetics, and in-vivo antitumor efficacy. Eur J Pharm Biopharm. 2022;179:106297. [Link] [DOI:10.1016/j.ejps.2022.106297]
18. Mohammadi M, Hamishehkar H, Piruzifard MK. Nanophytosome as a promising carrier for improving cumin essential oil properties. Food Biosci. 2021;42:101079. [Link] [DOI:10.1016/j.fbio.2021.101079]
19. Moradi-Kor N, Ghanbari A, Rashidipour H, Yousefi B, Bandegi AR, Rashidy-Pour A. Beneficial effects of Spirulina platensis, voluntary exercise and environmental enrichment against adolescent stress induced deficits in cognitive functions, hippocampal BDNF and morphological remolding in adult female rats. Horm Behav. 2019;112:20-31. [Link] [DOI:10.1016/j.yhbeh.2019.03.004]
20. Moradi-Kor N, Dadkhah M, Ghanbari A, Rashidipour H, Bandegi AR, Barati M, et al. Protective effects of spirulina platensis, voluntary exercise and environmental interventions against adolescent stress-induced anxiety and depressive-like symptoms, oxidative stress and alterations of BDNF and 5HT-3 receptors of the prefrontal cortex in female rats. Neuropsychiatr Dis Treat. 2020;16:1777-94. [Link] [DOI:10.2147/NDT.S247599]
21. Moutinho ILD, Maddalena NCP, Roland RK, Lucchetti ALG, Tibiriçá SHC, Ezequiel ODS, et al. Depression, stress and anxiety in medical students: A cross-sectional comparison between students from different semesters. Rev Assoc Med Bras. 2017;63:21-8. [Link] [DOI:10.1590/1806-9282.63.01.21]
22. Wahed WYA, Hassan SK. Prevalence and associated factors of stress, anxiety and depression among medical Fayoum University students. Alexandria J Med. 2017;53(1):77-84. [Link] [DOI:10.1016/j.ajme.2016.01.005]
23. Aloufi MA, Jarden RJ, Gerdtzm MF, Kapp S. Reducing stress, anxiety and depression in undergraduate nursing students: Systematic review. Nurse Educ Today. 2017;102,104877 [Link] [DOI:10.1016/j.nedt.2021.104877]
24. Hormozi M, Mirzaei R, Nakhaee A, Izadi S, Dehghan Haghighi J. The biochemical effects of occupational exposure to lead and cadmium on markers of oxidative stress and antioxidant enzymes activity in the blood of glazers in tile industry. Toxicol Ind Health. 2018;34(7):459-67. [Link] [DOI:10.1177/0748233718769526]
25. Omari Z, Kazunori S, Sabti M, Bejaoui M, Hafidi A, Gadhi C, et al. Dietary administration of cumin-derived cuminaldehyde induce neuroprotective and learning and memory enhancement effects to aging mice. Aging (Albany NY). 2021;13(2):1671-85. [Link] [DOI:10.18632/aging.202516]









